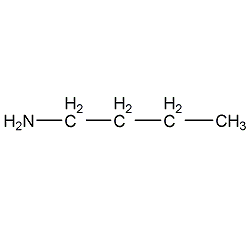
Structural formula
| Business number | 02ZM |
|---|---|
| Molecular formula | C4H11N |
| Molecular weight | 73.14 |
| label |
n-butylamine, 1-Aminobutane, 1-Amino-butane, n-Butylamine, herbicide, pesticides, Anti-glue agent, additive, antioxidants, Polymerization inhibitor |
Numbering system
CAS number:109-73-9
MDL number:MFCD00011690
EINECS number:203-699-2
RTECS number:EO2975000
BRN number:605269
PubChem ID:None
Physical property data
1. Properties: colorless and transparent liquid with ammonia smell. [1]
2. Melting point (℃): -50[2]
3. Boiling point (℃): 78[3]
4. Relative density (water = 1): 0.74[4]
5. Relative vapor Density (air=1): 2.52[5]
6. Saturated vapor pressure (kPa): 10.9 (20℃)[6]
7. Heat of combustion (kJ/mol): -3018.4[7]
8. Critical temperature (℃): 251[8]
9. Critical pressure (MPa): 4.16[9]
10. Octanol/water partition coefficient: 0.97 [10]
11. Flash point (℃): -12[11]
12. Ignition temperature (℃): 310[12]
13. Explosion upper limit (%): 9.8[13]
14. Explosion lower limit (% ): 1.7[14]
15. Solubility: Miscible with water, miscible with ethanol and ether. [15]
16. Viscosity (mPa·s, 25ºC): 0.681
17. Heat of generation (KJ/mol, 25ºC, liquid): -127.82
18. Heat of formation (KJ/mol, 25ºC, gas): -94.2
19. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.57
20.pKa (20ºC, water): 10.777
Toxicological data
1. Acute toxicity[16]
LD50: 366mg/kg (rat oral); 430mg/kg (mouse oral Oral); 629mg/kg (rabbit transdermal)
LC50: 800mg/m3 (mouse inhalation, 2h)
2. Irritation[17]
Rabbit transdermal: 500 mg, toxic stimulation (open stimulation test).
Rabbit eye: 250mg (24h), severe irritation.
Ecological data
1. Ecotoxicity[18]
LC50: 24~32mg/L (96h) (fish); 30~70ppm (24h ) (Water flea, static)
EC50: 43mg/L (24h) (Water flea)
2. Biodegradability [19] Using general activated sludge treatment, the removal rates within 5d, 10d, 15d, and 50d are 26.5%, 48.8%, 50%, and 52.3% respectively. Using activated sludge treatment adapted to the aniline environment, the removal rates within 6 days and 12 days were 50% and 67% respectively.
3. Non-creative�Degradability[20] In the air, when the concentration of hydroxyl radicals is 5.00×105 pieces/cm3, The degradation half-life is 11h (theoretical).
Molecular structure data
1. Molar refractive index: 24.11
2. Molar volume (cm3/mol): 98.2
3. Isotonic specific volume (90.2K ): 220.3
4. Surface tension (dyne/cm): 25.3
5. Polarizability (10-24cm3): 9.56
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 13.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: It has the chemical properties of primary amines. The aqueous solution is alkaline and can undergo photolysis (100°C) and pyrolysis (650~950°C). Butylamine can generate dibutylamine and tributylamine through a copper catalyst with pumice as a carrier at 260~270℃.
2. Daily exposure to 15~30mg/m3 butylamine vapor will cause nose, throat, eye irritation and headache, and facial skin will become red. At 30~75mg/m3, it becomes unbearable within a few minutes. Severe primary irritation and second-degree burns may occur on skin contact with abutamine liquid. The maximum allowable concentration in the workplace is 20.65 mg/m3.
3. Stability[21] Stable
4. Incompatible substances[22] Strong oxidants, acids, aluminum
5. Polymerization hazard[23] No polymerization
Storage method
1. Storage precautions [24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, and aluminum, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. This product is flammable and can form an explosive mixture when in contact with air. Packed in glass bottles and fixed with wooden frames, each bottle is 12kg. Beware of container leaks. Store in a cool and ventilated place. Fireworks are strictly prohibited and should be stored isolated from oxidants.
Synthesis method
1. Butanol amination method: n-butanol vapor and ammonia are reacted under normal pressure at 170-200°C through heated alumina, molybdenum oxide and other catalysts to generate a butylamine mixture, and then the product is After distillation and separation, one, two and tributylamine products can be obtained.
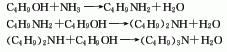
2. Butanol chlorination, Amination method: Add ethanol, ammonia and chlorobutane into the autoclave, stir and raise the temperature to 85-95°C, the pressure is about 0.54-0.64MPa, keep it for 6 hours, cool and reduce the pressure. Heat the reaction solution to recover ammonia, then add hydrochloric acid to pH=3-4, then recover ethanol, add liquid caustic soda to the crude liquid to pH 11-12, separate the upper liquid, and collect the fractions below 95°C through distillation to obtain n-Butylamine finished product, yield 50%. Raw material consumption quota: chlorobutane (80%) 3295kg/t, ethanol (95%) 840kg/t, ammonia (20%) 1500kg/t, liquid ammonia 546kg/t, hydrochloric acid (30%) 1170kg/t, liquid alkali (30%) 4515kg/t, solid alkali 1670kg/t. In addition, this product can also be directly prepared by reacting n-butane chloride with ammonium hydroxide in ethanol.
![]()
3. Combine butanol and ammonia and hydrogen through a Cu-Ni catalyst with clay as a carrier at 170-200°C to obtain a mixture of three amines, namely n-butylamine, di-n-butylamine and tri-n-butylamine. The product is fractionated to obtain n-butylamine, di-n-butylamine and tri-n-butylamine. Products of butylamine and tri-n-butylamine. Reaction equation: C4H9OH+NH3[H2]→C4H9NH2[C4H9OH]→(C4H9)2NH[C4H9OH]→(C4 H9)3N
Or use butanol as raw material, react with hydrogen chloride to generate chlorobutane, and combine ammonia-containing ethanol and ammonia water and chlorobutane are pressed into the autoclave, stir and heat up to 85~95°C, the pressure is 539~637kPa, keep for 6 hours, cool, and the reaction is completed. Heat the reaction solution to recover ammonia, then add hydrochloric acid to pH=3~4, and then recover Ethanol, add alkali solution to this crude liquid until pH=11~12, separate the upper liquid, and collect the fraction below 95°C through distillation as n-butylamine product. Reaction equation: C4H9OH+HCl→C4H9Cl+H2 O
C4H9Cl+NH3[C2H5OH]→CH3(CH2)3NH2+HCl
Purpose
1. Used in the manufacture of medicines, dyes, pesticides, emulsifiers, preservatives, petroleum product additives, flotation agents, special soaps, etc. Also used in the rubber industry and color photography industry.
2. Used in the preparation of pesticides, herbicides and drugs to treat diabetes.
3. Used as intermediates and chemical reagents in the manufacture of emulsifiers, pharmaceuticals, pesticides, rubber products, and dyes. [25]
�Manufacturing. Also used in the rubber industry and color photography industry.
2. Used in the preparation of pesticides, herbicides and drugs to treat diabetes.
3. Used as intermediates and chemical reagents in the manufacture of emulsifiers, pharmaceuticals, pesticides, rubber products, and dyes. [25]

 微信扫一扫打赏
微信扫一扫打赏

