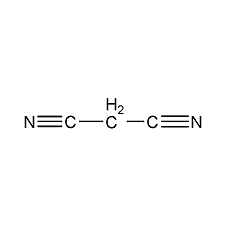
Structural formula
| Business number | 02ZR |
|---|---|
| Molecular formula | C3H2N2 |
| Molecular weight | 66 |
| label |
dicyanomethane, dicyanomethane, methylene cyanide, Malonitrile, Cyanomethine, Cyanoethane |
Numbering system
CAS number:109-77-3
MDL number:MFCD00001883
EINECS number:203-703-2
RTECS number:OO3150000
BRN number:773697
PubChem number:24896644
Physical property data
1.Character: colorless to yellow crystal[1]
2. Melting point (℃): 32[2]
3. Boiling point (℃): 220[3]
4. Relative density (water=1): 1.191[4]
5. Saturated vapor pressure (kPa): 2.67 (109°C) [5]
6. Heat of combustion (kJ/mol): -1650.3 [6]
7. Critical pressure (MPa): 3.6[7]
8. Octanol/water distribution Coefficient: -0.6[8]
9. Flash point (℃): 112[9]
10. Ignition temperature (℃): 590[10]
11. Explosion limit (%): 19[11]
12. Lower explosion limit (%): 2.9[12]
13. Solubility: soluble in water, ethanol, benzene, slightly soluble in chloroform and acetic acid. [13]
14. Refractive index: 1.4146 (34.2℃)
Toxicological data
1. Acute toxicity[14]
LD50: 14mg/kg (rat oral); 19mg/kg (mouse Oral); 350mg/kg (rat transdermal)
2. Irritation [15] Rabbit transdermal: 5mg (24h), Severe irritation.
3. Subacute and chronic toxicity [16] Rat abdominal cavity, 2mg/(kg·d), later 4, 28mg/(kg ·d), for a total of 19 weeks (total dose 228 mg/kg), no poisoning symptoms or accumulation effects were found.
Ecological data
1. Ecotoxicity[17]
LC50: 1.6mg/L (96h) (rainbow trout)
2. Biodegradability No data yet
3. Non-biodegradability[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 476d (theoretical).
When the pH value is 5, 6, 7, 8, and 9, its hydrolysis half-life is 21.4d, 21.3d, 20.2d, 13.4d, 3d (theoretical) respectively.
Molecular structure data
1. Molar refractive index: 15.77
2. Molar volume (cm3/mol): 64.8
3. Isotonic specific volume (90.2K ): 168.0
4. Surface tension (dyne/cm): 45.2
5. Polarizability (10-24cm3): 6.25
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 47.6
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 82.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[19] Stable
2. Incompatible substances[20] Strong oxidants, strong reducing agents, strong acids, strong bases
3. Polymerization hazards[21] Polymerization
Storage method
1. Storage precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, reducing agents, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. This product is packed in 50kg or 200kg iron-plastic drum, which is moisture-proof. Store in a cool, ventilated special warehouse away from fire and heat sources; store and transport in isolation from oxidants, acids and edible raw materials. In particular, high heat should be avoided and strictly isolated from alkaline substances.
Synthesis method
1. Laboratory produced by the elimination reaction of cyanoacetamide and phosphorus oxychloride. Stir and mix 1260g (15mol) cyanoacetamide, 1kg salt and 5L dichloroethane for 15 minutes, then add 800ml (8.75mol) phosphorus oxychloride. Subsequently, the mixture was heated to reflux for 8 hours. Cool to room temperature and filter. Wash the filter cake with 50 ml dichloroethane. Combine the filtered and washed liquids, and evaporate the solvent to obtain crude malononitrile. The crude product was distilled under reduced pressure, yielding 113-118°C (3.3kPa) fraction to obtain 570-654g of product, with a yield of 57-66%.
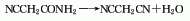
2. Allene Oxidation solution.
![]()
3. Reaction of acetonitrile and cyanogen chloride Law. Currently, Chinese companies use the following production methods: ethyl cyanoacetate (methyl ester) → amination → elimination → distillation → refining → malononitrile.
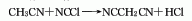
4. By the action of ethyl cyanoacetate and ammonia , react below 20°C, cool in an ice bath for 2 hours, filter out the precipitate, wash the filter cake with ice water, and then recrystallize with ethanol to obtain cyanoacetamide, which is reacted with phosphorus pentoxide and reduced pressure. Distill, collect fractions below 110~120℃ as crude products, and then distill under reduced pressure to obtain finished products.
NCCH2COOC2H5+NH3→NCCH2CONH2+C2H5OH
NCCH2CONH2+P2O5→NC—CH2—CN+2HPO3
Purpose
1. Used in organic synthesis, gold leaching agent. In medicine, it is used to synthesize a series of important drugs such as vitamin B1, triamterene, and triamterene. It has important uses in dyes, pesticides and other aspects. It can also be used as an extraction agent for gold. China is currently mainly used to produce triamterene, bensulfuron, 1,4,5,8-naphthalenetetracarboxylic acid and pyrimidine series products.
2. Used in organic synthesis and as a leaching agent for gold. [23]

 微信扫一扫打赏
微信扫一扫打赏

