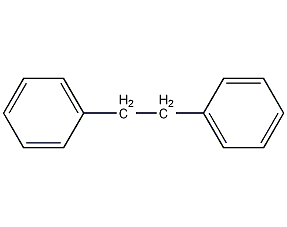
Structural formula
| Business number | 02N8 |
|---|---|
| Molecular formula | C14H14 |
| Molecular weight | 182.26 |
| label |
1,2-Diphenylethane, 1,2-Diphenylethane, Nitrocellulose solvent, impregnating agent for electric power containers, Dye solvent for carbonless copy paper, plastic plasticizer, Aromatic hydrocarbons |
Numbering system
CAS number:103-29-7
MDL number:MFCD00004796
EINECS number:203-096-4
RTECS number:DT4375000
BRN number:508068
PubChem number:24848578
Physical property data
1. Appearance: white monoclinic prism, needle or flake crystal
2. Density (g/mL, 25/4℃): 0.9782
3 . Melting point (ºC): 50~53
4. Boiling point (ºC, normal pressure): 284
5. Flash point (ºC): >110
6. Solubility: Easily soluble in carbon disulfide, ether, chloroform, amyl acetate, soluble in ethanol and liquid sulfur dioxide, almost insoluble in water and liquid ammonia.
7. Relative density (25℃, 4℃): 0.958360
8. Crystal phase standard hot melt ( J·mol-1·K-1): 253
9. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -7561.49
10. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): 51.55
11. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -7652.87
12. Gas phase standard claimed heat (enthalpy) (kJ·mol -1): 142.93
13. Refractive index (n60D):1.5476
Toxicological data
Acute toxicity: Rat oral LD50:4518mg/kg; MousePeritoneal cavityLD50:2500 mg/kg; “font-family:宋体;FONT-SIZE: 9pt”>Mice were intravenously injected with LD50:78mg/kg; >LD50:>5mg/kg;
Ecological data
Usually not harmful to water.
Molecular structure data
1. Molar refractive index: 60.28
2. Molar volume (cm3/mol): 182.9
3. Isotonic specific volume (90.2K ): 456.0
4. Surface tension (dyne/cm): 38.5
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 23.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 120
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants. White needle-like or small flake crystals. Boiling point: Easily soluble in chloroform, ether, carbon disulfide and amyl acetate, soluble in alcohol, and almost insoluble in water. It reacts with chromium trioxide or permanganic acid to generate benzoic acid.
2. Found in oriental tobacco leaves.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction between benzyl chloride and metallic sodium. Benzyl chloride reacts with metallic sodium at 160-180°C for 4 hours to obtain bibenzyl. Another method is derived from the reaction of benzyl chloride in the presence of copper, or the hydrogenation of benzoin in the presence of nickel.
2. Tobacco: OR, 26
3. Preparation method:
Day_121107/201211071508527463.gif “alt =” “/>
In the reaction bottle with a reflux condenser (with a calcium chloride dry pipe), add the radius chlorine (2) 100g (0.78 mol), 24g (1.0mol) of lump metal sodium, heated in oil bath to 160~180%. Heat the reaction for 4 hours. Change to a distillation device for atmospheric distillation. Distill the distillate again and collect the fraction at 273-276°C to obtain 20g of diphenylethane (1), which solidifies into colorless crystals after cooling. After recrystallization in ethanol, mp52℃.[1]
Purpose
Used as a solvent for organic synthesis and nitrocellulose. It is used as an impregnating agent for electric power containers and as a dye solvent for carbonless copy paper. It can also be used as a plastic plasticizer and a high-temperature heating medium.

 微信扫一扫打赏
微信扫一扫打赏

