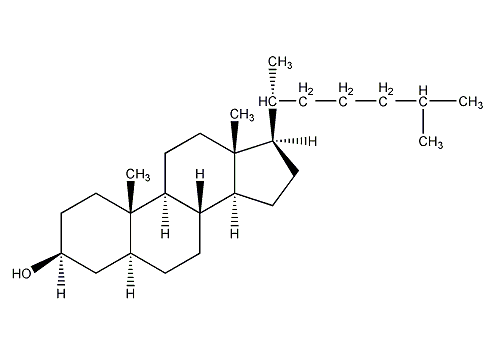
Structural formula
| Business number | 01RN |
|---|---|
| Molecular formula | C27H48O |
| Molecular weight | 388.68 |
| label |
β-Cholestanol, 3β-Hydroxy-5α-cholestane, 5α-Cholestan-3β-ol, Dihydrocholesterol |
Numbering system
CAS number:80-97-7
MDL number:MFCD00066413
EINECS number:201-315-8
RTECS number:FZ6350000
BRN number:2418594
PubChem number:2418594
Physical property data
1. Properties: The solution is colorless and transparent.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 138~140℃
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa) : Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): +28°±1°; +24°±1° (c=1, chloroform)
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil-water (octanol/water) distribution Log value of coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: dissolve 1g in 10ml chloroform
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 120.35
2. Molar volume (cm3/mol): 406.1
3. Isotonic specific volume (90.2K ): 987.0
4. Surface tension (dyne/cm): 34.8
5. Polarizability (10-24cm3): 47.71
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 9.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 5
5. Topological molecular polar surface area (TPSA): 20.2
6. Number of heavy atoms: 28
7. Surface charge: 0
8. Complexity���540
9. The number of isotope atoms: 0
10. The number of determined atomic stereocenters: 9
11. The number of uncertain atomic stereocenters : 0
12. Determined number of stereocenters of chemical bonds: 0
13. Uncertain number of stereocenters of chemical bonds: 0
14. Covalent bond unit Quantity: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
1. It is obtained by hydrogenation of cholesterol both in vivo and in vitro. It can also be synthesized from fecal sterol. It is present in trace amounts with cholesterol in vertebrate cells and is abundant in nervous tissue and adrenal glands. Dihydrocholesterol in the body can come from external sources or can be synthesized by itself. It can be synthesized by all tissues, but mainly in the liver.
2.
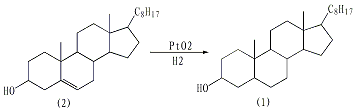 First, remove the crude cholesterol (2) 100g (0.26mol) is recrystallized with 250mL glacial acetic acid. The pure product obtained does not need to be dried and is directly added to the hydrogenation device. Add 300mL glacial acetic acid and 0.5g platinum oxide. Hydrogenate at 65-75℃ under slight pressure. After about 2 hours, hydrogen gas is basically no longer absorbed. After slightly cooling, the reaction mixture was filtered, concentrated under reduced pressure, and crystallized to obtain 85-90 g of crude dihydrocholesterol (containing part of cholesteryl acetate). Add the crude product to 400 mL of ethanol, then mix it with a dilute alkali made of 25 g of potassium hydroxide and 100 mL of water, and react under reflux for 3 hours. Cool, filter, and wash with water. The filter cake was recrystallized with 500 mL of ethanol to obtain 75-80 g of dihydrocholesterol, mp 140-141°C, and a yield of 75%-80%. [1]
First, remove the crude cholesterol (2) 100g (0.26mol) is recrystallized with 250mL glacial acetic acid. The pure product obtained does not need to be dried and is directly added to the hydrogenation device. Add 300mL glacial acetic acid and 0.5g platinum oxide. Hydrogenate at 65-75℃ under slight pressure. After about 2 hours, hydrogen gas is basically no longer absorbed. After slightly cooling, the reaction mixture was filtered, concentrated under reduced pressure, and crystallized to obtain 85-90 g of crude dihydrocholesterol (containing part of cholesteryl acetate). Add the crude product to 400 mL of ethanol, then mix it with a dilute alkali made of 25 g of potassium hydroxide and 100 mL of water, and react under reflux for 3 hours. Cool, filter, and wash with water. The filter cake was recrystallized with 500 mL of ethanol to obtain 75-80 g of dihydrocholesterol, mp 140-141°C, and a yield of 75%-80%. [1]
Purpose
The role in the body is not yet clear, it may be related to the formation of bile acid and certain hormones

 微信扫一扫打赏
微信扫一扫打赏

