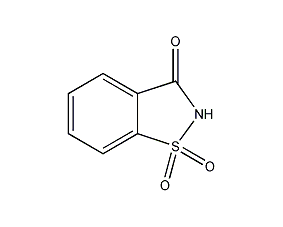
Structural formula
| Business number | 01RR |
|---|---|
| Molecular formula | C7H5NO3S |
| Molecular weight | 183 |
| label |
o-Sulfonylbenzene(methane)imide, insoluble saccharin, Benzosulfimide, Saccharin |
Numbering system
CAS number:81-07-2
MDL number:MFCD00005866
EINECS number:201-321-0
RTECS number:DE4200000
BRN number:6888
PubChem number:24847625
Physical property data
1. Properties: White monoclinic crystal. Vacuum sublimation produces needle-like crystals.
2. Density (g/mL, 25/4℃): 0.828g/cm3
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 228.8~229.7℃
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Not sure Confirm
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturation Vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (% , V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Slightly soluble in water, ether and chloroform, Soluble in ethanol, ethyl acetate, acetone. Its sodium salt is called saccharin sodium or soluble saccharin, which is easily soluble in water. The sweetness of the dilute aqueous solution is about 300 to 500 times that of sucrose
Toxicological data
Toxicity classification Low toxicityAcute toxicity Oral – mouse LD50; 17000 mg/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 41.91
2. Molar volume (cm3/mol): 117.6
3. Isotonic specific volume (90.2K ): 325.4
4. Surface tension (dyne/cm): 58.6
5. Polarizability (10-24cm3): 16.61
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 63.2
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 303
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Also known as o-sulfonyl benzene imide. Its sodium salt is called saccharin sodium or soluble saccharin, which is easily soluble in water. The sweetness of the dilute aqueous solution is about 300 to 500 times that of sucrose. Non-toxic in small amounts, but has no nutritional value. Colorless to white crystals or white crystalline powder, odorless or slightly aromatic. It tastes extremely sweet, and the aqueous solution diluted 10,000 times still has a sweet taste. The sweetness of its dilute solution is about 500 times that of sucrose, and its aftertaste is slightly bitter. Melting point: 228°C (decomposition). Saturated aqueous solution 0.25%) is acidic (pH 2.0). It is very unstable (decomposes) under acidic conditions (pH value below 3.8). It can be decomposed when heated with alkali or its aqueous solution (alkali is more stable than acid). Slightly soluble in water (25°C, 1g/290ml; boiling water, 1g/25m1) and soluble in alkaline solutions (ammonia, alkali hydroxide, alkali carbonate, etc.). Slightly soluble in ethanol (1g/31m1), slightly soluble in chloroform and ether.
Storage method
This product should be stored in a sealed, cool, dry place away from light.
Synthesis method
The preparation method is to obtain phthalic anhydride through amination degradation, diazotization, substitution, oxidation, acid precipitation and other steps. 1. Amination, degradation and esterification to prepare methyl anthranilate. 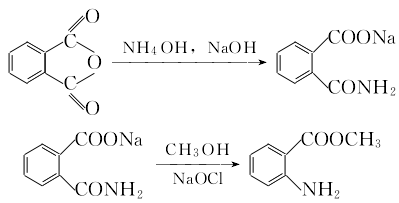 Add phthalic anhydride to the reaction pot, add 0°C ammonium hydroxide solution, raise the temperature to 50°C, and add For sodium hydroxide solution, control the alkali addition rate to keep the temperature below 70°C and the pH value at 8.5 to 8.9. After adding alkali, adjust the pH value to 12~13, keep it at 65~70°C for 0.5h, keep it warm for 35h to drive out ammonia gas, cool to -10°C, add pre-cooled -10°C methanol and -10°C sodium hypochlorite The solution should be reacted below 0℃ for 45 minutes, and then gradually raised to 30℃. It should show a colorless reaction when tested with potassium iodide starch test paper, and then add an appropriate amount of sodium bisulfite for hydrolysis. After the material liquid becomes dilute, heat it to 50°C, then add 80°C hot water, stir and dissolve, let it stand, filter, and separate the oil layer to obtain methyl anthranilate.
Add phthalic anhydride to the reaction pot, add 0°C ammonium hydroxide solution, raise the temperature to 50°C, and add For sodium hydroxide solution, control the alkali addition rate to keep the temperature below 70°C and the pH value at 8.5 to 8.9. After adding alkali, adjust the pH value to 12~13, keep it at 65~70°C for 0.5h, keep it warm for 35h to drive out ammonia gas, cool to -10°C, add pre-cooled -10°C methanol and -10°C sodium hypochlorite The solution should be reacted below 0℃ for 45 minutes, and then gradually raised to 30℃. It should show a colorless reaction when tested with potassium iodide starch test paper, and then add an appropriate amount of sodium bisulfite for hydrolysis. After the material liquid becomes dilute, heat it to 50°C, then add 80°C hot water, stir and dissolve, let it stand, filter, and separate the oil layer to obtain methyl anthranilate.
2. Preparation of formyl o-sulfonylbenzoate through heavy amination, substitution and chlorination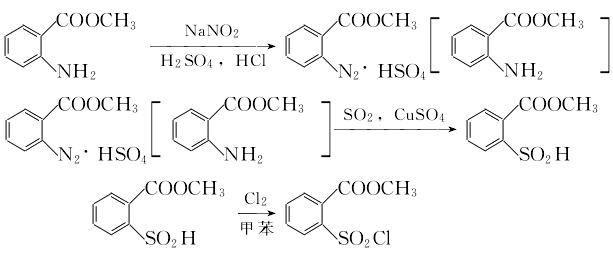 Stir the methyl anthranilate and sodium nitrite solutions evenly, then add them dropwise to the mixed acid in the diazo pot for reaction. The starting temperature of the dripping is about 10°C, the reaction temperature does not exceed 25°C, and the end point of the reaction is the potassium iodide starch solution. It is light green. Cool the diazo reaction solution to 10°C, add copper sulfate to dissolve it, and then pass in SO2 (approximately 1 hour) to precipitate methyl o-sulfenic acid benzoate. Use H acid test paper to test the end point of the substitution and it should be colorless. Add toluene. , vent chlorine gas to 2% at 30-35°C. The benzidine ethylamine test paper test shows dark dark green as the end point of chlorination. Let it stand and separate the layers. Take the organic layer to obtain a toluene solution of methyl o-sulfonyl chloride benzoate. 3. Cyclization and acid precipitation to obtain insoluble saccharin
Stir the methyl anthranilate and sodium nitrite solutions evenly, then add them dropwise to the mixed acid in the diazo pot for reaction. The starting temperature of the dripping is about 10°C, the reaction temperature does not exceed 25°C, and the end point of the reaction is the potassium iodide starch solution. It is light green. Cool the diazo reaction solution to 10°C, add copper sulfate to dissolve it, and then pass in SO2 (approximately 1 hour) to precipitate methyl o-sulfenic acid benzoate. Use H acid test paper to test the end point of the substitution and it should be colorless. Add toluene. , vent chlorine gas to 2% at 30-35°C. The benzidine ethylamine test paper test shows dark dark green as the end point of chlorination. Let it stand and separate the layers. Take the organic layer to obtain a toluene solution of methyl o-sulfonyl chloride benzoate. 3. Cyclization and acid precipitation to obtain insoluble saccharin 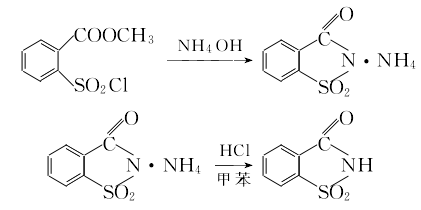 Mix water and methyl o-sulfonyl chloride benzoate toluene Add the solution to the reaction pot, cool to 10°C, add ammonium hydroxide solution and stir, the temperature reaches a maximum of 70°C, and test the endpoint after the pH value is above 9 for 15 minutes (2% benzidine pyrazole test paper, if it does not immediately show yellow, the endpoint is the endpoint). Take the layered ammonium salt solution and add toluene and 30% hydrochloric acid to make the pH=1 or less. After acid precipitation, cool to 20°C, separate the acid water layer and the toluene layer and wash with water to remove the ammonium chloride to obtain insoluble saccharin toluene colloid.
Mix water and methyl o-sulfonyl chloride benzoate toluene Add the solution to the reaction pot, cool to 10°C, add ammonium hydroxide solution and stir, the temperature reaches a maximum of 70°C, and test the endpoint after the pH value is above 9 for 15 minutes (2% benzidine pyrazole test paper, if it does not immediately show yellow, the endpoint is the endpoint). Take the layered ammonium salt solution and add toluene and 30% hydrochloric acid to make the pH=1 or less. After acid precipitation, cool to 20°C, separate the acid water layer and the toluene layer and wash with water to remove the ammonium chloride to obtain insoluble saccharin toluene colloid.
Purpose
Saccharin is o-benzoylsulfonimide, which is an intermediate between the fungicide allylbenthiazole and the herbicides metsulfuron-methyl, bensulfuron-methyl, fensulfuron-methyl, and chlorsulfuron-methyl.
Mainly used for the production of pesticide intermediates and the production of inhibitors of saccharin sodium phosphotransferase and phosphohydrolase. Test for thallium. Non-caloric sweetener. Electroplating brightener formula. Pharmaceutical aids. Used as a curing accelerator for anaerobic adhesives. In the electroplating industry, it is used as a brightener for plating tweezers, which can make the coating crystallize finely and have a relatively uniform gloss.

 微信扫一扫打赏
微信扫一扫打赏

