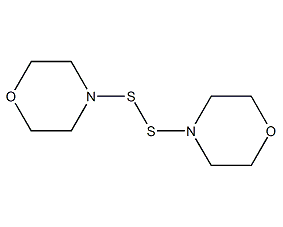
Structural formula
| Business number | 02ND |
|---|---|
| Molecular formula | C8H16N2O2S2 |
| Molecular weight | 236.36 |
| label |
N,N’-bisdithiobis(morpholine), Vulcanizing agent MD, N,N’-Dimorpholine Disulfide, N,N’-Dithiobis(morpholine), vulcanizing agent MD, Rubber vulcanizers and accelerators |
Numbering system
CAS number:103-34-4
MDL number:MFCD00023319
EINECS number:203-103-0
RTECS number:QE3325000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: White needle-like crystals. It has a fishy smell.
2. Density (g/mL, 20℃): 1.32~1.38
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 124-125
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20.2ºC): Not determined
12. Saturation Vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in benzene, carbon tetrachloride, Slightly soluble in acetone and gasoline, hardly soluble in ethanol and ether, and insoluble in water.
Toxicological data
1. Acute toxicity: Rat pass 50: 4300mg/kg; the mouse passing LD50: 1660mg/kg; the mouse inhaled LC50: 1624mg/m3; the mouse peritoneal ld50: 50 mg/kg; the mouse intravenous injection LD50: 100mg/kg; 2. Other multiple dose toxicity: Rat oral TDLo: 5375mg/kg/43W-I; Rat inhalation TCLo: 200 mg/m3/24H/17W-C; 3. Mutagenicity: Mutant microorganism test : Bacteria-Typhimurium�Monella, 100μg/plate; DNA repair test: bacteria-Escherichia coli, Bacillus subtilis, 1mg/disc;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 62.27
2. Molar volume (cm3/mol): 177.4
3. Isotonic specific volume (90.2K ): 489.5
4. Surface tension (dyne/cm): 57.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 24.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 75.5
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 143
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Decomposes when encountering inorganic acid or inorganic alkali. Stable when stored at room temperature. Non-toxic, has a fishy smell. Contact with skin or mucous membranes can cause a strong and lasting acrid sensation. Protective equipment should be worn during operation.
Storage method
Tightly sealed in wooden barrels lined with plastic bags. Good storage stability at normal storage temperature. Should be protected from sun and moisture.
Synthesis method
Add morpholine, solvent gasoline (or benzene and toluene) and a small amount of water to the reaction kettle. After stirring evenly, add sulfur monochloride, gasoline and sodium hydroxide solution dropwise into the kettle at the same time, and control the temperature at 10°C. Next, the sodium hydroxide solution should be added dropwise before the carbon monochloride. After the dropwise addition is completed, add a certain amount of water and continue stirring for 30 minutes. The reactants are then suction filtered, and the filtrate is separated into gasoline and water phases and the gasoline is recovered. The filter cake is added to the centrifuge and washed with water until neutral. After the water is removed, it is dried to obtain the finished product.

Purpose
This product can be used as vulcanizing agent and accelerator for natural rubber and synthetic rubber. When used as a vulcanizing agent, active sulfur can be decomposed only at the vulcanizing temperature, so it is safe to use and no scorch will occur. The vulcanization speed of this product is slow when used alone, but when used in combination with accelerators such as thiazoles, thiurams or dithiocarbamates, the vulcanization speed can be increased. Although acidic substances such as salicylic acid can promote the decomposition of this product and speed up the vulcanization speed, they can also reduce the physical properties of the vulcanized rubber. When used as an accelerator, due to the high effective sulfur content of this product, the dosage of sulfur can be appropriately reduced. When using this product, the glue will not bloom, pollute or discolor. When used in effective and semi-effective vulcanization systems, the vulcanized rubber obtained has good heat resistance and aging resistance. This product is easily dispersed in rubber materials. When used as a vulcanizing agent alone, the general dosage is 3 to 4 parts; when used as an accelerator, the general dosage is 0.5 to 2 parts, and combined with 0.5 to 2 parts of sulfur.

 微信扫一扫打赏
微信扫一扫打赏

