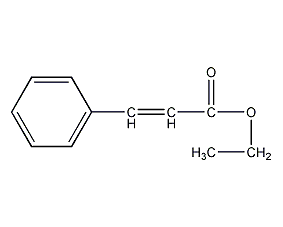
Structural formula
| Business number | 02NE |
|---|---|
| Molecular formula | C11H12O2 |
| Molecular weight | 176.21 |
| label |
3-Phenylethyl acrylate, Ethyl Phenylacrylate, Ethyl cinnamate, Cinnamic acid Ethyl ester, Ethyl trans-3-Phenylacrylate, trans-3-Phenylacrylic acid ethyl ester, Ethyl phenylacrylate, Fragrance fixative, modifier, spices |
Numbering system
CAS number:103-36-6
MDL number:MFCD00009189
EINECS number:203-104-6
RTECS number:GD9010000
BRN number:1238804
PubChem number:24890292
Physical property data
1. Properties: Colorless oily liquid
2. Relative density (g/mL, 20/4ºC): 1.0491
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point: 12℃ (6.5~7.5ºC)
5. Boiling point (ºC, normal pressure): 271.5
6. Boiling point (ºC, normal pressure) 2.0KPa): 144
7. Refractive index (20ºC): 1.5598
8. Flash point (ºC): 93.5
9. Viscosity (mPa· s, 20ºC): 8.7
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Vapor pressure (kPa, 87.6ºC): 0.13
13. Heat of evaporation (KJ/mol): 58.6
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, soluble in water Miscible with ethanol and ether.
Toxicological data
1. Acute toxicity: Rat oral LD50: 4000mg/kg; mouse oral LC50: 4000mg/kg;
Rabbit skin contact LD50: >5000mg/kg; Guinea pig oral LD50: 4000mg/kg;
2. Other multiple dose toxicity: Rat oral TDLo: 9520mg/kg/17W-I;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 53.18
2. Molar volume (cm3/mol): 166.9
3. Isotonic specific volume (90.2K): 414.5
4. Surface tension (dyne/cm): 38.0
5. Dielectric constant:
6. Dipole moment (10-24cm3 ):
7. Polarizability: 21.08
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 26.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 179
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It has the fragrance and background of sweet orange and grape, and the aroma is long-lasting. It has the properties of a general ester and is hydrolyzed under the action of caustic alkali. Because it contains double bonds, it is prone to polymerization under the action of light and heat. When stored in a dark place for a long time, it emits rose red fluorescence, turns green when illuminated by transmitted light, and produces white insoluble powder.
Storage method
Pay attention to fire sources, especially avoid sunlight, and store in a cool and sealed place.
Synthesis method
1. Cinnamic acid method: Obtained from the esterification of cinnamic acid and ethanol in the presence of sulfuric acid.
2. Benzaldehyde method: obtained by reacting benzaldehyde with ethyl acetate.
3. Preparation method:
Add 1.0mmol triphenylethoxycarbonyl methylenephosphonium ylide, 1.0mmol benzaldehyde, and 2g silica gel (200~300 mesh) into the reaction bottle ). Place the reaction bottle in a 400W microwave reactor and heat for 5 minutes. After cooling, add 30 mL of methylene chloride to extract, concentrate, and purify on a silica gel column to obtain 150 mg of almost colorless oily liquid ① (1), with a yield of 85%. Note: ① The following compounds can be synthesized using a similar method (Table I-9-2, page 500 of the reference book). [1]
Purpose
This product exists in natural styrax and has a fruit-like aroma with a clear and sweet breath. It has a better effect when combined with fragrant perilla oil and natural fruit essential oil. It is especially suitable for use in powder, perfume and essence. It can also be used in edible bayberry essence. This product is also used in organic synthesis.
It is used in the spice industry to blend fragrant perilla oil and citrus oil, to prepare cologne and oriental floral essences, and as a flavor fixative. It can also be used in edible bayberry essence. Can be used as a general reagent.

 微信扫一扫打赏
微信扫一扫打赏

