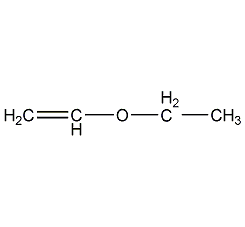
Structural formula
| Business number | 0305 |
|---|---|
| Molecular formula | C4H8O |
| Molecular weight | 72.11 |
| label |
Ethyl Vinyl Ether, Ethoxyethylene, vinyl ether, vinyl ethyl ether, Ethyleneoxyethane, 1-Ethoxyethene, Vinyl ethyl ether, Anesthetic, analgesics, softener, adhesive, surface treatment agent, synthetic rubber improver, Electronic coating raw materials and intermediates |
Numbering system
CAS number:109-92-2
MDL number:MFCD00009248
EINECS number:203-718-4
RTECS number:KO0710000
BRN number:605351
PubChem ID:None
Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 20/20℃): 0.754
3. Relative vapor density (g/mL, air =1): 2.5
4. Melting point (ºC): -115
5. Boiling point (ºC, 101.3kPa): 35.5
6. Refractive index (n20ºC): 1.3767
7. Refractive index (n25ºC): 1.3739
8. Flash point (ºC): <17.8
9. Viscosity (mPa ·s, 20ºC): 0.22
10. Flash point (ºC): 201.7
11. Vapor pressure (kPa, 20ºC): 56.80
12. Vapor Pressure (kPa,-49.0ºC): 1.33
13. Critical temperature (ºC): 201.85
14. Critical pressure (MPa): 4.07
15 . Body expansion coefficient (K-1, 55ºC): 0.00165
16. Body expansion coefficient (K-1, 10~40ºC): 0.00102
17. Explosion upper limit (%, V/V): 28.0
18. Explosion lower limit (%, V/V): 1.7
19. Dissolution Properties: Slightly soluble in water, miscible with a variety of organic solvents such as acetone, benzene, ether, heptane, methanol, carbon tetrachloride, etc. Dissolves 0.9% in water at 20°C; water dissolves 0.2% in ethyl vinyl ether.
20. Relative density (20℃, 4℃): 0.7589
21. Eccentricity factor: 0.467
22. Solubility parameter (J·cm-3)0.5: 16.125
23. van der Waals area (cm2·mol-1): 7.010×109
24. van der Waals volume (cm3·mol-1 ): 4g src=”http://images.basechem.org/internal/day_110120/201101201556039070.gif” alt=”” />
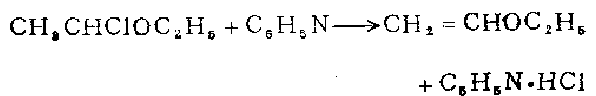
Preparation of ethyl vinyl ether
At room temperature, 24 g (0.22 mol) of 1-chloroethyl ethyl ether was added to 40 ml (0.5 mol) of ethyl ether. During distillation, 14 ml of distillate can be collected before the steam temperature reaches 100°C. The distillate was then fractionated through a 1 cm long pine needle-shaped fractionating column to obtain ethyl vinyl ether, weighing 6.8 g (43%).
3. Preparation method:
![]()
In a reaction flask equipped with a fractionating column (connected to a condenser and cooled by an ice-salt bath), add 50g of absolute ethanol (1.08mol) and 5g of mercury acetate (0.016mol) , 100g (1.0mol) of n-butyl vinyl ether (2), heated to reflux. Control the temperature at the top of the fractionating column at 36-37°C, and steam out 71g of ethyl vinyl ether (1) with a yield of 98%. Add water to the distillation bottle, perform azeotropic distillation (97°C), and separate to obtain 71.2g of n-butanol, with a yield of 97%. [1]
Purpose
1. Used as chemical intermediates, spices and lubricant additives, etc. Used in softeners, adhesives, coatings, leather coatings, textile surface treatment agents, synthetic rubber improvers and intermediates in the manufacture of sulfadiazine.
2. Vinyl ether paralyzes the central nervous system, and its anesthetic effect is stronger than ether, so it is used as an anesthetic and analgesic in medicine. This product is also an intermediate for fine chemicals and can be used in the production of the drug sulfadiazine, the disinfectant glutaraldehyde, polymers, coatings, etc. It can also be used to prepare spices and lubricating oil additives.
3. Ethyl vinyl ether is mainly used as a protecting group for hydroxyl groups, a vinyl transfer reagent, and in cycloaddition reactions in organic synthesis.
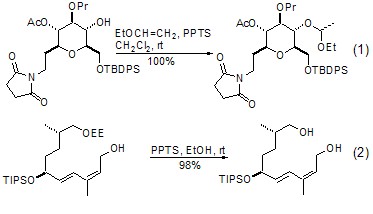
Among the many hydroxyl protective groups, α-ethoxyethyl ether (EE) generated from ethyl vinyl ether and hydroxyl group is the most commonly used and convenient protection group. One of the bases. This reaction generally requires a strong acidic catalyst, TFA and TsOH can be used for this purpose, the most commonly used is PPTS. This reaction can usually be completed by stirring at room temperature for several hours. In most cases, the yield of the product is more than 95% (Formula 1)[1,2]. The EE protecting group can easily complete the deprotecting reaction under acidic conditions. PPTS-EtOH and aq. HCl-MeOH are recommended methods (Formula 2)[3,4].
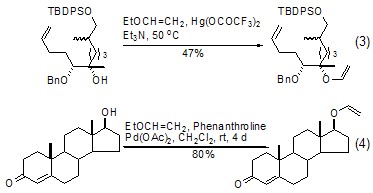
In the catalyst of mercury acetate derivatives Under the action of ethyl vinyl ether and hydroxyl group, a vinyl transfer reaction occurs to generate the corresponding vinyl ether product (formula 3)[5]. If a metal palladium catalyst is used, the use of a metal mercury catalyst (Formula 4)[6] can be effectively avoided. This reaction is more meaningful because the product can undergo further condensation reactions or olefin metathesis[7,8].
Ethyl vinyl ether and olefin The vinyl transfer reaction of propyl hydroxyl group produces the corresponding vinyl ether product. The reaction is usually completed under the catalysis of mercury acetate. Heating the product in toluene or xylene can induce the corresponding Claisen rearrangement, making this reaction of important synthetic value. Normally, the two-step reaction process can be completed under “one pot cooking” conditions (Formula 5, Formula 6)[9~11].
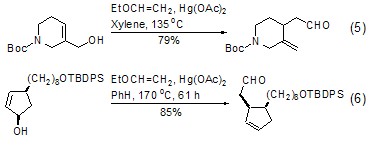
In the presence of Lewis acid catalyst , ethyl vinyl ether can also undergo corresponding cyclization reactions[12,13].

 微信扫一扫打赏
微信扫一扫打赏

