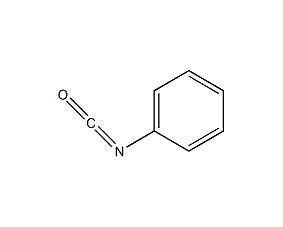
Structural formula
| Business number | 02P2 |
|---|---|
| Molecular formula | C7H5NO |
| Molecular weight | 119.11 |
| label |
Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:103-71-9
MDL number:MFCD00001994
EINECS number:203-137-6
RTECS number:DA3675000
BRN number:471391
PubChem number:24887343
Physical property data
1. Properties: colorless to light yellow liquid with pungent odor. [1]
2. Melting point (℃): -30[2]
3. Boiling point (℃): 166[3]
4. Relative density (water = 1): 1.1[4]
5. Saturated steam Pressure (kPa): 0.25 (20℃)[5]
6. Critical pressure (MPa): 4.54[6]
7. Octanol/water partition coefficient: 2.59[7]
8. Flash point (℃): 51[8]
9. Ignition temperature (℃): 601[9]
10. Solubility: Easily soluble in ether. [10]
Toxicological data
1. Acute toxicity: rat oral LD50: 800mg/kg; rat inhalation LC50: 22mg/m3/4H; rat skin contact LD50: 5mL/kg; rat peritoneal cavity LD50: 100mg/kg; small Mouse oral LD50: 196mg/kg; Mouse peritoneal cavity LD50: 50mg/kg; Rabbit skin contact LD50: 7130mg/kg;
2. Other multiple dose toxicity: rat inhalation TCLo: 7mg/m3 /6H/2W-I; Rat inhalation TCLo: 500ppb/6H/3W-I;
3. Mutagenicity: Mutant microorganism test: bacteria-Salmonella typhimurium, 100μg/plate.
4. Acute toxicity [11] LD50: 940mg/kg (rat oral); 7130mg/kg (rabbit transdermal)
p>
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[12] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 7.5d (theoretical).
4. Other harmful effects [13] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 36.15
2. Molar volume (cm3/mol): 118.4
3. Isotonic specific volume (90.2K ): 295.2
4. Surface tension (dyne/cm): 38.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.33
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 29.4
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 121
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocentersNumber of �� bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[14] Stable
2. Incompatible substances[15] Water, alcohols, strong alkalis, amines, acids, strong oxidants
3. Conditions to avoid contact[16] Exposure to heat and humid air
4. Polymerization hazard[17] No polymerization
5. Decomposition products[18] Hydrogen cyanide
Storage method
1. Storage precautions [19] Store in a cool, dry, and well-ventilated special warehouse, and implement the “two people to send and receive, and two people to keep” system. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Dangerous Regulation No. 61109, small opening steel drum; wooden crates outside screw-top glass bottles, iron-top pressure-top glass bottles, plastic bottles or metal drums; plastic bottles, tin-plated thin steel drums outside Bottom lattice box. Store in a cool, ventilated warehouse. Keep away from fire, heat sources and direct sunlight. Keep container tightly sealed. Pay attention to moisture and rain, and store it separately from oxidants, acids, and food chemicals.
Synthesis method
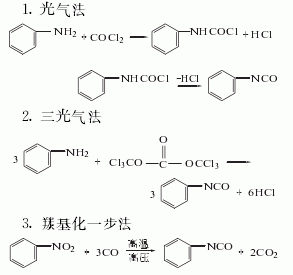
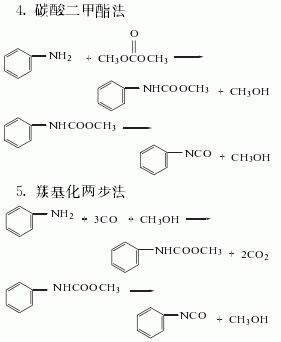
Among the above five methods, the dimethyl carbonate method is the only Methanol is produced, and methanol is the raw material for the synthesis of dimethyl carbonate. It is a clean production process. In addition, hydrochloric acid is produced as a by-product in the triphosgene method. my country has now introduced hydrochloric acid electrolysis technology, which can form a closed loop for this method. It is also a potential production method.
6. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and ventilation tube (extending into the bottom of the bottle), add aniline (2) 93g (1.0mol), 1L of toluene, and stir in dry hydrogen chloride gas until saturated to generate aniline hydrochloride. Heat to 70°C and add phosgene until the reaction solution becomes clear. Then fractionate, and collect the fractions at 158-163°C to obtain 99g of phenyl isocyanate (1), with a yield of 83%. [21]
Purpose
Used to identify alcohols and amines, to prepare pesticides, and as intermediates in organic synthesis. [20]

 微信扫一扫打赏
微信扫一扫打赏

