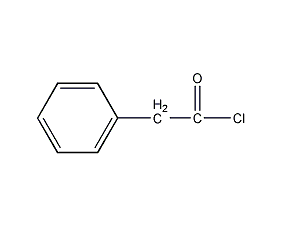
Structural formula
| Business number | 02P8 |
|---|---|
| Molecular formula | C8H7ClO |
| Molecular weight | 154 |
| label |
phenylacetyl chloride, Acetyl chloride, phenyl |
Numbering system
CAS number:103-80-0
MDL number:MFCD00000729
EINECS number:203-146-5
RTECS number:None
BRN number:742254
PubChem number:24887236
Physical property data
1. Properties: colorless to light yellow fuming liquid. [1]
2. Boiling point (℃): 94~95 (1.6kPa) [2]
3. Relative density (water = 1): 1.17[3]
4. Saturated vapor pressure (kPa): 0.13 (48℃)[4]
5. Octanol/water partition coefficient: 1.240[5]
6. Flash point (℃): 102[6]
7. Solubility: Easily soluble in ether. [7]
8. Refractive index: 1.5325
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 40.68
2. Molar volume (cm3/mol): 130.7
3. Isotonic specific volume (90.2K ): 327.3
4. Surface tension (dyne/cm): 39.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 116
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[8] Stable
2. Incompatible substances[9] Strong oxidants, strong alkalis, water, alcohols
3. Conditions to avoid contact[10] Humid air
4. Polymerization hazard[11] No polymerization
5. Decomposition products[12] Hydrogen chloride, phosgene
Storage method
Storage Precautions[13] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and protected from moisture. Respond to oxygenStore chemicals, alkalis, and food chemicals separately, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Prepared from the reaction of phenylacetic acid and thionyl chloride. Mix equal moles of phenylacetic acid and spicy thionyl chloride and react at room temperature to release sulfur dioxide and hydrogen chloride gas. When a large amount of gas is no longer released, heat the reaction mixture to 30-40°C, keep it warm for 25 hours, and add a quarter of the volume of benzene to the mixture. Use low-temperature distillation to remove volatile substances, and gradually reduce the pressure to 0.133kPa. After all volatile substances are removed, steam out the 55-57°C (0.133kPa) fraction, which is phenylacetyl chloride. The yield is 80-85%.
Purpose
1. Used in spice preparation, organic synthesis, and also used as experimental reagents. [14]

 微信扫一扫打赏
微信扫一扫打赏

