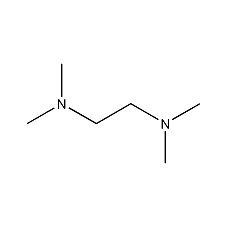
Structural formula
| Business number | 030R |
|---|---|
| Molecular formula | C6H16N2 |
| Molecular weight | 116.21 |
| label |
Tetramethylethylenediamine, Tetramethylethylenediamine, 1,2-bis(dimethylamino), 1,2-bis(dimethylamino)ethane, tetramethyl-1,2-ethylenediamine, N,N,N’,N’-methylethylenediamine, 1,2-Bis(dimethylamino)ethane, linear compound |
Numbering system
CAS number:110-18-9
MDL number:MFCD00008335
EINECS number:203-744-6
RTECS number:KV7175000
BRN number:1732991
PubChem number:24900591
Physical property data
1. Characteristics: colorless transparent liquid with slight ammonia smell. [1]
2. Melting point (℃): -55[2]
3. Boiling point (℃): 120~122[3]
4. Relative density (water=1): 0.77 (20℃)[4]
5. Relative vapor density (air=1): 4.0[5]
6. Octanol/water partition coefficient: 0.3[6]
7. Flash point (℃): 10[7]
8. Ignition temperature (℃): 349[8]
9. Explosion upper limit (%): 9.08[9]
10. Explosion lower limit (%): 0.98[ 10]
11. Solubility: miscible with water, miscible with ethanol and most organic solvents. [11]
Toxicological data
1. Irritation: Rabbit skin open irritation test: 10mg/24H; Rabbit eye standard Drez eye dye test: 750ug severe irritation
2. Acute toxicity: Rat oral LD50: 268mg/kg; rat inhalation LC50: 1318ppm/4H; mouse oral LD50: 630mg/kg; rabbit skin LD50: 5390mg/kg; rabbit subcutaneous LD50: 1230mg/kg; quail oral LD50: >316mg/kg;
3. Other multiple dose toxicity: rat inhalation TCLO: 250ppm/6H/11D-I;
4. Acute toxicity[12]
LD50: 268mg/kg (rat oral); 5390mg/kg (rabbit transdermal)
LC50: 1318ppm (rat inhalation, 4h)
p>
5. Irritation[13]
Rabbit transdermal: 10mg (24h), causing irritation (open stimulation test ).
Rabbit eye: 750μg, severe irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information yet
4. Other harmful effects [14] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 37.28
2. Molar volume (cm3/mol): 142.0
3. Isotonic specific volume (90.2K ): 322.2
4. Surface tension (dyne/cm): 26.4
5. Polarization�� (10-24cm3): 14.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 6.5
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 42.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[15] Stable
2. Incompatible substances[16] Strong oxidants, strong acids
3. Conditions to avoid contact[17] Heat
4 .Polymerization hazard[18] No polymerization
5. Decomposition products[19] Amine p>
Storage method
Storage Precautions[20] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Put 4060 ml of 90% formic acid in a 12-liter three-neck round-bottomed flask equipped with a condenser, stirrer and dropping funnel. Slowly add 1040 grams (17.3 mol) of ethylene glycol from the dropping funnel while stirring. amine, then quickly add 5960 ml of 37% formaldehyde solution. Uniform evolution of carbon dioxide from the solution indicates that the reaction is in progress. When the initial phase of rapid bubble evolution subsides, heat to reflux for 48 hours. While still boiling, carefully treat the reaction mixture with 1.0 liters of concentrated sulfuric acid to salt the amine. Evaporate excess formaldehyde, formic acid and water. After collecting 5600 ml of distillate, the residue begins to foam, add a new 50% aqueous solution of 900 g of sodium hydroxide, and approximately 1 liter of amine product will be evaporated. Treatment of the residue with concentrated aqueous potassium hydroxide solution salts out more of the amine. Combine the obtained amines, dry them with granular potassium hydroxide, and distill them together with sodium to obtain N,N,N’N’-tetramethylethylenediamine.
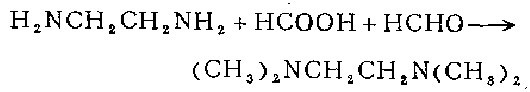
Purpose
Used as biochemical reagents, epoxy resin cross-linking agents, and intermediates for the preparation of quaternary ammonium compounds. [21]

 微信扫一扫打赏
微信扫一扫打赏

