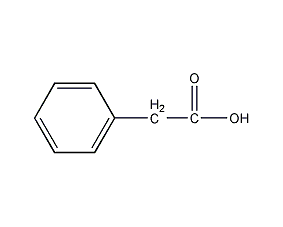
Structural formula
| Business number | 02PA |
|---|---|
| Molecular formula | C8H8O2 |
| Molecular weight | 136.15 |
| label |
Phenyl acetic acid, phenylalanine, Alpha-toluic acid, Phenylacetic acid, Acidephenylacetique, Benzenacetic acid, Kyselina fenyloctova, Pesticide intermediates; aromatic carboxylic acids and their derivatives, acidic solvent |
Numbering system
CAS number:103-82-2
MDL number:MFCD00004313
EINECS number:203-148-6
RTECS number:AJ2430000
BRN number:1099647
PubChem ID:None
Physical property data
1. Properties: White powder, with a special odor, slightly sour, and a civet-like turbid and floral undertone. The aroma is strong and the fragrance is very long-lasting.
2. Density (g/mL, 77℃): 1.081
3. Relative vapor density (g/mL, air=1): ~4
4. Melting point (ºC): 77.5
5. Boiling point (ºC, normal pressure): 265.5
6. Boiling point (ºC, 1.33×10-3kPakpa): 65-70
7. Relative density (25℃, 4℃): 1.09177.5
8. Flash point (ºC): 132
9. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -3892.8
10. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -397.61
11. Vapor pressure (mmHg, 97ºC): 1
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, soluble in ethanol, ether, ammonia, and also soluble in sodium carbonate and ammonia solutions, and miscible with grease and oily spices.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2250mg/kg;
Rat peritoneal cavity LD50: 1600mg/kg;
Mouse oral LD50: 2250mg/kg;
Mouse peritoneal cavity LD50: 2270mg/kg;
&n3mol), water 200mL. Sodium hydroxide 40g (1mol), heated to reflux for 1.5h. Cool and acidify with hydrochloric acid until it becomes acidic to the red test paper. The precipitated solid was filtered, washed with a small amount of cold water, and dried to obtain 33 g of phenylacetic acid (1), with a yield of 82%. Phenylacetic acid is recrystallized from water to obtain colorless flake crystals. [2]
5. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and dropping funnel, add 4.8g (0.2mol) clean magnesium chips and one grain of iodine, and slowly add benzyl chloride (2) Prepare 20 mL of a solution of 25.4 g (0.2 mol) and 80 mL of anhydrous ether. Heat slowly to start the reaction. Keep boiling and add the rest of the solution dropwise. After the addition was completed, the reaction was continued with reflux and stirring for 0.5 h. Cool in an ice water bath, add dry carbon dioxide gas, and the reaction will be exothermic. After about 2 hours of passage, the reaction temperature dropped. The surface reaction is over. Add 50g of ice water to the reaction solution, and adjust it with dilute hydrochloric acid until it becomes acidic to the Congo red test paper. The ether layer was separated, and the aqueous layer was extracted once with ether. Combine the ether layers and extract the ether layer with excess sodium hydroxide solution. The ether layer was separated, and the aqueous layer was acidified with dilute hydrochloric acid to precipitate crude phenylacetic acid. After suction filtration and recrystallization with water, 16 g of phenylacetic acid (1) was obtained, mp78°C, yield 59%. [3]
Purpose
1. Phenylacetic acid is an intermediate in organic synthesis of medicines, pesticides, spices, etc.
2. Used in the production process of penicillin to increase the total output of penicillin G and used as a raw material for the preparation of spices. It can also be used in baked goods, meat products, and sweet sauces.
3. Can be used in tobacco flavors, especially in Havana cigar flavor.

 微信扫一扫打赏
微信扫一扫打赏

