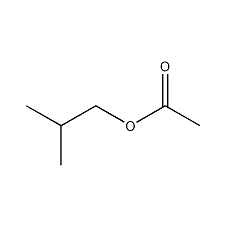
Structural formula
| Business number | 030S |
|---|---|
| Molecular formula | C6H12O2 |
| Molecular weight | 116.16 |
| label |
2-Methylpropyl acetate, Isobutyl acetate, Butyl isoacetate, 2-Methylpropyl ethanoate, food additives, Flavor enhancer |
Numbering system
CAS number:110-19-0
MDL number:MFCD00008932
EINECS number:203-745-1
RTECS number:AI4025000
BRN number:1741909
PubChem number:24890060
Physical property data
1. Properties: colorless and transparent liquid with fruity aroma. [1]
2. Melting point (℃): -98.9[2]
3. Boiling point (℃): 116.5[3]
4. Relative density (water = 1): 0.87[4]
5. Relative vapor Density (air=1): 4.0[5]
6. Saturated vapor pressure (kPa): 2.37 (25℃)[6]
7. Heat of combustion (kJ/mol): -3533.8[7]
8. Critical temperature (℃): 296[8]
9. Critical pressure (MPa): 3.24[9]
10. Octanol/water partition coefficient: 1.78 [10]
11. Flash point (℃): 17.8 (CC); 29.4 (OC) [11]
12. Ignition temperature (℃): 423[12]
13. Explosion limit (%): 10.5[13]
14. Lower explosion limit (%): 1.3[14]
15. Solubility: Slightly soluble in water, miscible in ethanol and ether. [15]
16. Viscosity (mPa·s, 20ºC): 0.697
17. Flash point (ºC, closed): 17.8
18. Flash point (ºC, open): 31.1
19. Heat of evaporation (KJ/mol): 35.87
20. Heat of generation (KJ/mol): 506.60
21. Heat of combustion (KJ/mol): 3539.5
22. Specific heat capacity (KJ/(kg·K), 70.2ºC, constant pressure): 2.11
23. Electrical conductivity (S/m, 25ºC): 2.55×10-4
24. Volume expansion coefficient (K-1 ,55ºC): 0.00126
25. Critical density (g·cm-3): 0.290
26. Critical volume (cm3·mol-1): 401
27. Critical compression factor: 0.257
28. Eccentricity factor: 0.455
29. Liquid phase standard hot melt (J·mol-1·K-1): 232.6
Toxicological data
1. Irritation: Rabbit skin open irritation test: 500mg, slight irritation; rabbit skin standard Dreze eye dye test: 500mg/24H, moderate irritation; rabbit eye standard Dreze eye dye test: 500mg/24H Moderate irritation;
2. Acute toxicity: rat oral LD50: 13400mg/kg; rat inhalation LCL0: 8000ppm/4H; rabbit oral LD50: 4763mg/kg; rabbit skin LD50: >17400mg /kg;
3. Low toxicity, but long-term contact with concentrated vapor should be avoided. Rats exposed to 99.75 mg/m3 of isobutyl acetate for 150 minutes will become anesthetized and 100% fatal. The maximum allowable concentration in the workplace is 1896 mg/m3.
4. Acute toxicity[16]
LD50: 13400mg/kg (rat oral); >17400mg/kg (rabbit transdermal)
5. Irritation[17]
Rabbit transdermal: 500mg, mild stimulation (open stimulation test).
Rabbit eye: 500mg (24h), moderate irritation.
Ecological data
1. Ecotoxicity[18] IC50: 205mg/L (72h) (algae)
2. Biodegradability No data yet
3. Non-biodegradability[19] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 70h (theoretical).
When the pH value is 7 and 8, the hydrolysis half-life is 3a and 122d respectively (theoretical).
4. Other harmful effects[20] This substance is harmful to the environment. Special attention should be paid to the pollution of water bodies. .
Molecular structure data
1. Molar refractive index: 31.57
2. Molar volume (cm3/mol): 131.4
3. Isotonic specific volume (90.2K ): 292.9
4. Surface tension (dyne/cm): 24.6
5. Polarizability (10-24cm3): 12.51
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 76.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: Similar to butyl acetate, the hydrolysis rate is slower than butyl acetate. It decomposes when heated to above 450°C to produce isobutylene, acetic acid, acetone, carbon dioxide and methane. It reacts with ammonia to form amides. It reacts with benzene in the presence of aluminum trioxide to form tert-butylbenzene. When the photochlorination reaction is carried out, a monochlorosubstituted ester is obtained, in which the 2-chlorosubstituted product and the 3-chlorosubstituted product are equal.
2. Stability[21] Stable
3. Incompatible substances[22] Strong oxidants, strong bases, strong acids
4. Polymerization hazards[23] No polymerization
Storage method
Storage Precautions[24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the esterification of isobutyl alcohol and acetic anhydride in the presence of sulfuric acid. Mix acetic anhydride and isobutyl alcohol, add sulfuric acid dropwise, cool slightly and then heat to reflux for 5-6 hours. Wash the reflux liquid with water 2-3 times, neutralize with sodium carbonate, wash with water until neutral, dry with calcium chloride, and The finished product is obtained by fractionation under reduced pressure on an oil bath.
Refining method: In addition to water, acetic acid and isobutanol, the impurities also contain a mixture of homologous isopine bodies. During refining, neutralize and wash with sodium bicarbonate or saturated aqueous sodium carbonate solution, then wash thoroughly with saturated aqueous sodium chloride solution, dry with anhydrous sodium sulfate or magnesium sulfate and then rectify.
2. Preparation method:
Preparation of peroxytrifluoroacetic acid solution: In a reaction bottle containing 50mL of methylene chloride, add 90% H2O2 8.2mL (0.3mol ), slowly add 50.8 mL (0.36 mol) of trifluoroacetic anhydride dropwise while cooling. After adding, stir thoroughly and set aside. In a reaction bottle equipped with a stirrer, dropping funnel, and reflux condenser, add 130g of dry and finely ground disodium hydrogen phosphate, 150mL of 20.0g (0.2mol) of dichloromethane methyl isobutyl ketone (2) , add the above-mentioned peroxytrifluoroacetic acid solution dropwise within 20 minutes with stirring. During the dropwise addition, the reaction is exothermic and refluxes violently. After the addition, continue to heat and reflux for 30 minutes. After cooling, insoluble salts were filtered off and washed with dichloromethane. Combine the filtrate and washing liquid, wash with 10% sodium carbonate solution, and dry over anhydrous sodium sulfate. After most of the solvent is evaporated under normal pressure, it is fractionated in a fractionation device equipped with a fractionation head. The reflux ratio is controlled and the fractions at 114-115.5°C are collected to obtain 19.5g of colorless transparent liquid (1) with a yield of 84%. [26]
Purpose
1. This product has the aroma of raw pear and raspberry, and is often used as fruit flavor for blending banana, pineapple, raspberry and pear flavors. Also used as a rose preparation. Used as a solvent for paints, nitrocellulose spray paints, and varnishes.
2. Used as a solvent and chemical reagent for nitrocellulose and lacquer, and for preparing spices. [25]

 微信扫一扫打赏
微信扫一扫打赏

