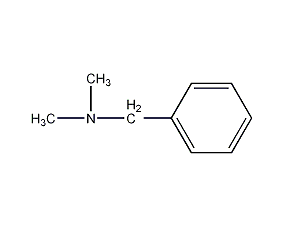
Structural formula
| Business number | 02PB |
|---|---|
| Molecular formula | C9H13N |
| Molecular weight | 135.21 |
| label |
N,N-dimethylbenzylamine, benzyldimethylamine, dimethylbenzylamine, N,N-dimethylbenzylamine, N-Benzyldimethylamine, benzyl dimethylamine, N-benzyldimethylamine, Tertiary amine curing agent, dehydrogenation catalyst, acid neutralizer, preservative |
Numbering system
CAS number:103-83-3
MDL number:MFCD00008329
EINECS number:203-149-1
RTECS number:DP4500000
BRN number:1099620
PubChem number:24848115
Physical property data
1. Properties: colorless to light yellow liquid with ammonia smell.
2. Density (g/mL, 27℃): 0.9000
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -75
5. Boiling point (ºC, normal pressure): 183~184
6. Boiling point (ºC, 2.4kPa): 65~68
7. Refractive index: 1.501
8. Flash point (ºC): 54
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC):
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V) : Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in cold water, soluble in hot water, miscible in Alcohol, ether.
Toxicological data
1. Skin/eye irritation:
Standard Dresser test: rabbit skin contact, 500mg/4HREACTION SEVERITY, strong reaction;
Standard Dresser test: rabbit Eye contact, 5mgREACTION SEVERITY, strong reaction;
2. Acute toxicity: Rat oral LD50: 265mg/kg;
Rat inhalation LCLo: 1200mg/m3/2H;
Mice inhaled LCLo: 1200mg/m3/2H;
Rabbit skin contact LD50: 1660mg/kg;
3. Other multiple dose toxicity: rat inhalation TCLo: 30mg/m3/4H/26W-I;
4. Rabbit The lowest lethal dose is LD 250mg/kg. It has a weak excitatory effect on sympathetic nerves, but cannot inhibit tuberculosis bacteria. It is highly irritating and corrosive to skin and mucous membranes, and is also highly sensitizing.
Ecological data
This substance may be harmful to the environment and it is recommended not to let it enter the environment.
Molecular structure data
1. Molar refractive index: 44.15
2. Molar volume (cm3/mol): 146.0
3. Isotonic specific volume (90.2K): 349.3
4. Surface tension (dyne/cm): 32.7
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 17.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 3.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 82.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, acids, acid chlorides, and carbon dioxide.
Exposure to air absorbs carbon dioxide and turns it into carbonates. Flammable and toxic. It is recommended that operators wear self-priming filter dust masks, safety glasses, anti-toxic substance penetration overalls, and rubber gloves.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants, acids, acid chlorides, carbon dioxide, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
(1) Obtained from the reaction of benzyl and dimethylamine. First add dimethylamine to the reaction pot, add benzyl chloride with stirring, and control the temperature below 25°C. After the dropwise addition is completed, stir the reaction at room temperature for 1 hour, then slowly increase the temperature to 55-60°C and continue the reaction for 6 hours. Then add liquid caustic soda and let it stand for layering. Take the oil layer, wash it with hot water, distill it under reduced pressure, and collect the 67-72°C (1.87kpa) fraction to obtain benzyldimethylamine. 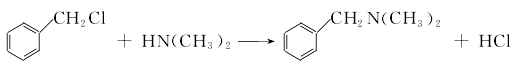 Raw material consumption: dimethylamine 2666kg/t; benzyl chloride 860kg/t. (2) Put 100kg of dimethylamine into the reaction kettle, slowly add 115kg of benzyl chloride dropwise under stirring and water bath cooling conditions, remove the cooling water after the dropwise addition, react at room temperature for 2 hours, and then heat to 50-60°C with steam. Reaction 5h. After the reaction, drain the water in the kettle, wash it twice with hot water, stop stirring after washing, cool it with circulating water, and drain the water after 40 hours. Collect the material phase to obtain the crude product. Distill the crude product under reduced pressure and collect the fractions with a gas phase temperature of 70-72°C at 1.6kpa to obtain chemically pure N,N-dimethylbenzylamine.
Raw material consumption: dimethylamine 2666kg/t; benzyl chloride 860kg/t. (2) Put 100kg of dimethylamine into the reaction kettle, slowly add 115kg of benzyl chloride dropwise under stirring and water bath cooling conditions, remove the cooling water after the dropwise addition, react at room temperature for 2 hours, and then heat to 50-60°C with steam. Reaction 5h. After the reaction, drain the water in the kettle, wash it twice with hot water, stop stirring after washing, cool it with circulating water, and drain the water after 40 hours. Collect the material phase to obtain the crude product. Distill the crude product under reduced pressure and collect the fractions with a gas phase temperature of 70-72°C at 1.6kpa to obtain chemically pure N,N-dimethylbenzylamine. 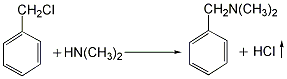
Purpose
This product is a commonly used tertiary amine curing agent for epoxy resin. The dosage is 6g/100g of resin, the service life is 4h, and the curing condition is 80℃ for 1h.
This product is also an organic synthesis intermediate and is used for the synthesis of quaternary ammonium salts and the drug chlormethionine, etc. It is also used as dehydrogenation catalyst, acid neutralizer, preservative and accelerator for electron microscope section embedding. Used as a curing agent for epoxy resin, the reference dosage is 10 to 15 parts by mass, and the curing condition is 80°C/1h. It is an important organic synthesis intermediate, such as the synthesis of quaternary ammonium salts. It is also used as a dehydrogenation catalyst, preservative, acid neutralizer, accelerator for electron microscope section embedding, etc.

 微信扫一扫打赏
微信扫一扫打赏

