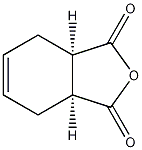
Structural formula
| Business number | 01VS |
|---|---|
| Molecular formula | C8H8O3 |
| Molecular weight | 152 |
| label |
Tetrahydrophthalic anhydride, 1,2,3,6-Tetrahydrophthalic anhydride, THPA, Aromatic carboxylic acids and their derivatives |
Numbering system
CAS number:85-43-8
MDL number:MFCD00005916
EINECS number:201-605-4
RTECS number:None
BRN number:82341
PubChem number:24848300
Physical property data
1. Properties: This product is white crystal
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/ mL, air=1): Undetermined
4. Melting point (ºC): 99~101
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 156
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16 . The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. The lower limit of explosion (%) ,V/V): Undetermined
19. Solubility: Soluble in general solvents, slightly soluble in petroleum ether
Toxicological data
1. Acute toxicity:
Rat inhalation LC: >294mg/kg; rat LD50: 3mg/kg;
Mouse inhalation LC: >294mg/kg; Mouse abdominal LD50: 500mg/kg;
Mouse LD50: 3300mg/kg;
Pig LD50: 3500mg/kg;
2. Neurotoxicity:
Rabbit skin test: 500 mg/24HREACTION; Rabbit eye test: 20 mg/24HREACTION; Rat oral administration
1, LD504590mg/kg Mice were intraperitoneally injected with LD50500mg/kg span>.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 36.41
2. Molar volume (cm3/mol): 118.0
3. Isotonic specific volume (90.2K): 305.5
4. Surface tension (dyne/cm): 44.9
5. Polarizability (10-24cm3): 14.43
Computing chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 43.4
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 218
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product has low toxicity. Contact with water generates acid, which is corrosive. When in contact with skin, wash immediately with soap and water.
Storage method
This product is sealed and stored in a dry place. Keep away from fire and heat sources, waterproof and moisture-proof.
Packaged in metal drums lined with plastic bags, 50kg per drum. Store in a cool and ventilated place, strictly protected from moisture. Store and transport flammable chemicals according to regulations and avoid open flames.
Synthesis method
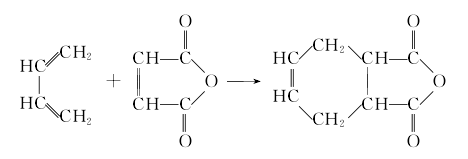
Add maleic anhydride into the reaction kettle, heat it until it is completely melted, stir and add solvent benzene, stir and mix, continue stirring and raise the temperature to 100-110°C, pass in butadiene for addition reaction 18- 22h, after the reaction is completed, the solvent benzene is evaporated, cooled by a condenser and then recycled for recycling. The reaction liquid is filtered and dried to obtain the finished product.
Purpose
1. 1,2,3,6-tetrahydrophthalic anhydride (tetrahydrophthalic anhydride for short, △4) is an intermediate for the preparation of the insecticide carbanthin and the fungicide captan It can also be used to prepare alkyd resin, unsaturated polyester resin, plasticizer and curing agent, etc. 2. Used as a curing agent for epoxy resin, the reference dosage is 75 to 85 parts by mass, and the curing condition is 130℃/ 16h or 200℃/1~2h, the thermal deformation temperature of cured product is 122℃. In the adhesive and coating industry, it is used to make light-colored alkyd resins, air-drying water-soluble alkyd resins, unsaturated polyester resins, solvent-free paints, and antifouling paints. It is also used to make plasticizers and stabilizers. , curing agent, used in the pesticide industry to produce the fungicide Captan and the insecticide pyrethroid.

 微信扫一扫打赏
微信扫一扫打赏

