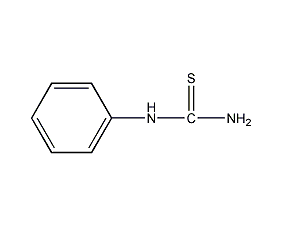
Structural formula
| Business number | 02PD |
|---|---|
| Molecular formula | C7H8N2S |
| Molecular weight | 152 |
| label |
N-Phenylthiourea, phenylthiocarbonamide, phenylthiourea, Phenylthiocarbonamide, 1-phenyl-2-thiourea, phenylthiourea, Benzene-2-thiourea, 1-Phenyl-2-thio-ure, alpha-Phenylthiourea, Fenylthiomocovina, Phenyl-thioure, aromatic sulfur compounds |
Numbering system
CAS number:103-85-5
MDL number:MFCD00004933
EINECS number:203-151-2
RTECS number:YU1400000
BRN number:907309
PubChem number:24898735
Physical property data
1. Properties: White needle-like crystals.
2. Density (g/mL, 20℃): 1.300
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 145-150
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, kPa): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20.2ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol, soluble in 400 parts of cold water , 17 parts in hot water.
Toxicological data
1. Acute toxicity: Rat oral LD50: 3mg/kg; Rat peritoneal cavity LD50: 5mg/kg; Mouse oral LD50: 10mg/kg; Mouse peritoneal cavity LD50: 25mg/kg; Rabbit oral LD50: 40mg/kg; 2. Biopharmaceuticals�Toxicity: Oral TDLo in mice 1-21 days after conception: 168mg/kgSEX/DURATION; 3. Mutagenicity: DNA damage test: rat liver, 8mmol/L;
Ecological data
General remarks
Do not allow this product to come into contact with groundwater, watercourses or sewage systems
Water hazard class 2 (German regulations) (self-assessment via list) This substance is hazardous to water Extremely harmful.
Even small amounts of product seeping into the ground can pose a hazard to drinking water.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 46.68
2. Molar volume (cm3/mol): 117.5
3. Isotonic specific volume (90.2K ): 341.7
4. Surface tension (dyne/cm): 71.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 70.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 119
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides, acids, and alkalis.
2.Eating, inhaling, entering the eyes, and being absorbed by skin contact will cause harm. Suitable protective clothing should be worn to avoid skin and eye contact. Combustion of this product produces irritating, corrosive and/or toxic gases.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acidic substances and alkali metals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. 2.Store in a cool, ventilated place and in an airtight container. Transportation must be labeled as “drugs” and transportation by air and rail is limited.
Synthesis method
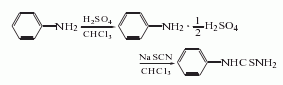
It is obtained by the addition reaction of aniline with sulfuric acid and then with sodium thiocyanide.
1. The sodium thiocyanide method first forms a salt with sulfuric acid and then performs an addition reaction with sodium thiocyanate. The reaction formula is as follows:
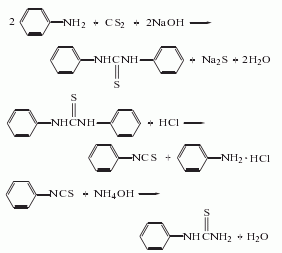
2. The diphenylthiourea method uses aniline and carbon disulfide to produce diphenylthiourea, which is then acidified and ammoniated. The reaction formula is as follows:
Purpose
Used to produce 2-aminobenzothiazole, 3-methylbenzothiazole hydrazone and other dye intermediates. Also used as analytical reagents.

 微信扫一扫打赏
微信扫一扫打赏

