Background and overview[1]
(3-Chlorophenyl)methylsulfonyl chloride is a sulfonyl chloride compound. Sulfonyl chlorides are a very important class of compounds, widely used as intermediates and raw materials in organic and pharmaceutical synthesis, and are an important source of sulfonyl groups. Due to the important role and widespread use of sulfonyl chloride, a variety of preparation methods for sulfonyl chloride have been developed. Generally speaking, alkylsulfonyl chlorides can be prepared by the following two methods: (1) direct chlorination of alkylsulfonic acid or its salt using chlorination reagents; (2) thiols and their precursors or derivatives ( Thiols, S-alkyl isothiouronium salts, disulfides, thiol acetates, thiol carbamates, etc.) are subjected to oxidative chlorination.
The first method has the disadvantages of harsh reaction conditions, long reaction time, low functional group tolerance, use of excessive chlorination reagents, and the generation of acidic or toxic by-products; although the second method has made great achievements in the past century Great progress has been made, but there are still shortcomings. For example, some oxidative chlorination reagents are not easy to obtain, some are dangerous to operate, some can cause heavy metal pollution, and some can produce organic by-products and interfere with the purification of the product. Therefore, how to prepare sulfonyl chloride simply becomes increasingly important.
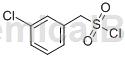
Preparation[1]
The preparation of (3-chlorophenyl)methylsulfonyl chloride is as follows: Dissolve thiourea (0.387g, 5mmol) and m-chlorobenzyl chloride (5mmol) in 5mL of ethanol, reflux for 30 minutes, and remove the solvent under reduced pressure. A white solid was obtained. The white solid was slowly added dropwise to a mixed system of NaClO2 (1.61g, 15mmol, 85% purity), concentrated HCl (3mL) and MeCN (10mL). During the feeding process, use a water bath to control the temperature in the reaction system between 10 and 20oC. After the addition is completed, continue to stir the reaction for 30 minutes, remove the acetonitrile under reduced pressure under low temperature conditions, add 25 mL of water, filter the solid in the system, and dry to obtain the product colorless crystal (3-chlorophenyl) methylsulfonyl chloride, melting point 76 ~ 78℃, 1.035g, yield 92%. 1HNMR (400MHz, CDCl3) δ: 7.48~7.37 (m, 4H), 4.83 (s, 2H).
Main reference materials
[1] CN201310276839.3 A general method for preparing sulfonyl chloride

 微信扫一扫打赏
微信扫一扫打赏

