Overview[1]
1-(p-Hydroxyphenyl)-2-thiourea is a pharmaceutical intermediate that can be prepared from benzoyl chloride in three steps.
Preparation[1]
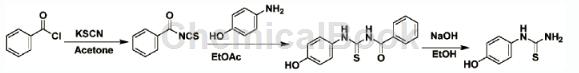
Add 12mmol benzoyl chloride and 20mL acetone into a 100mL round-bottomed flask, and then add 12mmol potassium thiocyanate. A large amount of white solid will be generated during the reaction. After 15 minutes, when the reaction is completed, the solid is filtered, and the filtrate is spun dry to obtain a light yellow liquid, which is directly put into the next step of the reaction. Add 10 mmol 4-hydroxyaniline to a 100 mL round-bottomed flask, dissolve 30 mL ethyl acetate, add the product obtained from the previous reaction to this solution, and heat to reflux reaction. After the TLC tracking reaction is completed, the reaction solution is cooled to room temperature, the solvent is evaporated under reduced pressure, 10 mL of ethanol and 10 mL of 2N sodium hydroxide solution are directly added, and the reaction is refluxed. After the TLC tracking reaction is completed, the reaction solution is cooled to room temperature, 30 mL of ice water is added, and neutralized with 2N hydrochloric acid until neutral. The solid was filtered to obtain the target product as a yellow solid with a yield of 89%. 1H NMR(400MHz, DMSO-d6)δ9.39(s,J2.6Hz,1H),9.38(s,1H),7.06(d,J=8.2Hz,2H),6.72 (d,J=8.7Hz, 2H).
Apply[1]
1-(p-Hydroxyphenyl)-2-thiourea can be used to prepare 2-(4-hydroxyphenylimino)-4-(4-fluorophenyl)thiazole. The test results of the antibacterial synergistic activity of the compound against polymyxin B show that the compound has significant antibacterial synergistic activity against polymyxin B.

The preparation operation is as follows: add 1mmol N-(4-hydroxyphenyl)thiourea and 1.05mmol α-bromo-4-fluoroacetophenone into a 25mL eggplant-shaped bottle, add 10mL ethanol to dissolve, and then add 1.5mmoL triethyl Amine, reflux reaction. After the TLC tracking reaction, the temperature of the reaction solution was lowered to room temperature, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (eluent: petroleum ether-ethyl acetate) to obtain the target compound as a white solid, with a yield of 85%. 1H NMR (400MHz, DMSO-d6) δ9.92 (s, 1H), 9.13 (s, 1H), 7.93 (dd, J = 8.7, 5.6Hz, 2H), 7.48 (d ,J=8.8Hz,2H),7.25(t,J=8.9Hz,2H),7.20(s,1H),6.76(d,J=8.8Hz,2H);19F NMR (376MHz, DMSO-d6 )δ-114.70 (tt,J=9.0,5.6Hz).
Main reference materials
[1] [Chinese invention] CN201610571725.5 Use of a thiazole compound as an antibacterial synergist

 微信扫一扫打赏
微信扫一扫打赏

