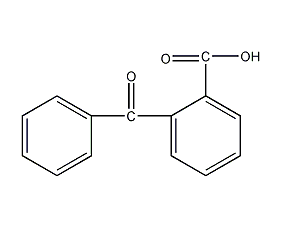
structural formula
| business number | 01vx |
|---|---|
| molecular formula | c14h10o3 |
| molecular weight | 226.23 |
| label |
benzophenone-2-carboxylic acid, benzophenone-2-carboxylic acid, aliphatic carboxylic acids and their derivatives |
numbering system
cas number:85-52-9
mdl number:mfcd00002472
einecs number:201-612-2
rtecs number:dg3600000
brn number:1107841
pubchem number:24891569
physical property data
1. properties: white triclinic needle crystals, industrial products are beige or white particles.
2. density (g/ml, 25/4℃): undetermined
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 127-129
5. boiling point (ºc, normal pressure): 257-265
6. boiling point (ºc, 5.2kpa) : undetermined
7. refractive index: undetermined
8. flash point (ºc): 257
9. specific rotation (º): undetermined determined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa, 25ºc): undetermined
12. saturated vapor pressure (kpa, 60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) partition coefficient: undetermined
17. explosion upper limit ( %, v/v): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: easily soluble in ethanol, ether, and in hot benzene and alkali solution, it precipitates in acid.
toxicological data
oral ld50 for rats: 400 mg/kg, and intraperitoneal injection of ld50 for mice: 562 mg/kg. production equipment should be sealed to prevent leakage. operators should wear protective equipment.
ecological data
molecular structure data
1. molar refractive index: 62.97
2. molar volume (cm3/mol): 180.0
3. isotonic specific volume (90.2k): 489.0
4. surface tension (dyne/cm): 54.3
5. polarizability (10-24cm3): 24.96
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 54.4
7. number of heavy atoms: 17
8. surface charge: 0
9. complexdegree: 292
10. number of isotope atoms: 0
11. determined number of atomic stereocenters: 0
12. uncertain atomic configuration number of centers: 0
13. determined number of stereocenters of chemical bonds: 0
14. uncertain number of stereocenters of chemical bonds: 0
15. covalent number of key units: 1
properties and stability
a needle-shaped crystal containing 1 molecule of water precipitated from water, with a melting point of 95°c. toxic and irritating to skin and mucous membranes.
storage method
store sealed in a cool, dry place. sealed packaging in iron drums, 170kg per drum. store in a cool, ventilated place. store and transport according to regulations on toxic substances.
synthesis method
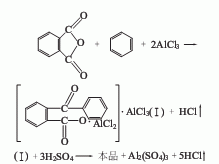
in the presence of anhydrous aluminum trichloride, it is obtained by condensing phthalic anhydride and benzene and then hydrolyzing it. the hydrochloric acid gas produced by the reaction should be absorbed with water into dilute hydrochloric acid for later use. industrial product benzoyl benzoic acid is a white to beige powder with a purity of over 97% and an initial melting point of dry product ≥ 126.0%. raw material consumption quota: phthalic anhydride 690kg/t, pure benzene (95%) 402kg/t, aluminum trichloride (98.5%) 1358kg/t, sulfuric acid (98%) 500kg/t.
purpose
this product is the main raw material for anthraquinone dye intermediates and is used to manufacture anthraquinone, benzanthrone, 1-aminoanthraquinone, etc.

 微信扫一扫打赏
微信扫一扫打赏

