Overview[1]
1-(3-methylphenyl)piperazine dihydrochloride is a pharmaceutical intermediate, which is the hydrochloride form of 1-(3-methylphenyl)piperazine. It can be synthesized from 1-(3 -Methylphenyl)piperazine is prepared from hydrogen chloride.
Preparation method[1]
The general route to prepare 1-(3-methylphenyl)piperazine is as follows. Reaction conditions: a.SOCl2, rt, 14-15h; b. 3-methylaniline, MW, 170℃-180℃, 30-45min
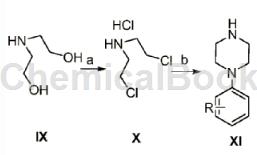
The specific operations are as follows:
Synthesis of (β-chloroethyl)amine hydrochloride (X)
In a 100 ml flask, dissolve diethanolamine (10 g) in chloroform (40 ml), and slowly add a solution of sulfoxide dichloride (2.6 ml) in chloroform (20 ml) at room temperature. React for 7 hours. When the reaction is complete, spin it to dryness, add additional chloroform, spin it to dryness again, and evaporate the thionyl chloride to dryness. After drying, 14 g of white solid IX was obtained, with a yield of 85%, mp: 214.1-215.2°C (literature value >215-216°C).
Synthesis of N-phenylpiperazine derivatives (XI)
Add 1.58g (10mmol) IX and 1mol aniline derivative into 10ml n-butanol, react under microwave at 180°C for 30 minutes. After the reaction is completed, let it stand and cool to crystallize. Filter, wash the filter cake with a small amount of methylene chloride, and dry it under vacuum to obtain white crystals (XI) with a yield of 45-54%.
Apply[1]
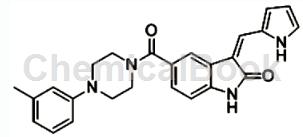
CN103467456 reported that the free base of 1-(3-methylphenyl)piperazine dihydrochloride can be used to synthesize 3,5-disubstituted indolinone derivatives such as: 3-((1H-pyrrole- 2-yl)methenyl)-5-(4-(m-methylphenyl)piperazine-1-carbonyl)indol-2-one (compound 30), this 3,5-disubstituted indolinone Derivatives can be used to prepare MAPK inhibitors and AKT inhibitors, thereby preparing anti-lung cancer drugs. The structure is as follows:
Main reference materials
[1] (CN103467456) 3,5-Disubstituted Indolinone Derivatives and Preparation Methods and Applications

 微信扫一扫打赏
微信扫一扫打赏

