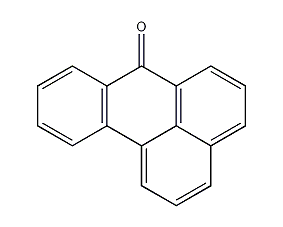
Structural formula
| Business number | 01SP |
|---|---|
| Molecular formula | C17H10O |
| Molecular weight | 230.26 |
| label |
7H-Benz[de]anthracen-7-one, 1,9-Benz-10-anthrone, Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:82-05-3
MDL number:MFCD00003585
EINECS number:201-393-3
RTECS number:CX5075000
BRN number:1455646
PubChem number:24846308
Physical property data
1. Properties: light yellow needle-like crystals
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/ mL, air=1): Uncertain
4. Melting point (ºC): 170
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain Determined
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical Temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
16. p>
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Dissolution Properties: Insoluble in water, soluble in ethanol and other organic solvents. Dissolves in sulfuric acid to form an orange liquid with red light and green fluorescence.
Toxicological data
1. Acute toxicity:
Rat abdominal LD50: 1500 mg/kg;
Mouse abdominal LD50: 290mg/kg;
2. Subacute toxicity: rat caliber TDL0: 15 gm/kg/30D-I; mouse skin LD50: 117 gm/kg/34W-I
3. Teratogenicity: human lymphocytes: 3300 ug /L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 71.76
2. Molar volume (cm3/mol): 178.9
3. Isotonic specific volume (90.2K ): 494.4
4. Surface tension (dyne/cm): 58.3
5. Polarizability (10-24cm3): 28.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Topological molecular polar surface area (TPSA) 17.1
6. Number of heavy atoms: 18
7. Surface charge: 0
8. Complexity: 348
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters: 0
11. Uncertain the number of atomic stereocenters: 0
12. Determine the number of chemical bond stereocenters: 0
13. The number of uncertain stereocenters of chemical bonds: 0
14. The number of covalent bond units: 1
Properties and stability
This product is toxic. If the human body comes into direct contact with it or operates in its dust, it will cause dermatitis, eczema and skin turning brown and black, and even cause adverse effects on the liver and stomach. Mice were intraperitoneally injected with LD50: 603mg/kg. The production workshop should have good ventilation, the equipment should be sealed, and operators should wear protective equipment.
Storage method
Store sealed in a cool, dry place.
Packaged in iron drums or cardboard drums, lined with plastic bags, with a net weight of 50kg or 100kg per drum. Store in a cool, dry place. Store and transport according to regulations on toxic chemicals.
Synthesis method
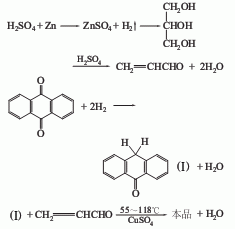
It is obtained by reacting anthraquinone and glycerol through the following steps. (1) Anthraquinone is reduced with iron powder in the presence of copper sulfate to form hydroxyanthrone. (2) Glycerol loses water and turns into acrolein under the action of concentrated sulfuric acid. (3) In the presence of sulfuric acid, hydroxyanthraquinone and acrolein add together and lose a molecule of water to form a new ring. (4) Then use sulfuric acid to oxidize to benzanthrone. Raw material consumption quota: anthraquinone (98%) 900kg/t, glycerol 600kg/t, zinc powder 440kg/t, copper sulfate (96%) 205kg/t, sulfuric acid (100%) 5000kg/t.
Purpose
Important dye intermediates, used to produce reduced brilliant green FFB, reduced olive green B, reduced olive T, reduced gray M, reduced black BBN, etc.

 微信扫一扫打赏
微信扫一扫打赏

