Background and overview[1]
Among chemical drugs, nucleosides and their derivatives with therapeutic effects play a very important role, and their numbers are growing rapidly. Among them, 1-acetoxy-2,3,5-trityl Acyloxy-1-beta-D-ribofuranose is a key intermediate in the synthesis of nucleosides and their derivatives. The chemistry and medicinal chemistry of nucleoside compounds is an emerging research field at home and abroad. Nucleosides or nucleotide analogs with antiviral activity are commonly known as nucleoside antiviral drugs and play an important role in the treatment of viral diseases. In addition, many nucleoside compounds also play an important role in anti-tumor. They have aroused widespread interest and great attention around the world, and related research articles are constantly being published.
1-Acetoxy-2,3,5-tribenzoyloxy-1-beta-D-ribofuranose is a nucleoside used to prepare the antiviral drug ribavirin or the antitumor drug fludarabine. Important intermediates of its derivatives. The methods currently reported in the literature for preparing 1-acetoxy-2,3,5-tribenzoyloxy-1-beta-D-ribofuranose have shortcomings such as long reaction steps, large environmental pollution, and high cost.
Preparation[1]
A method for preparing 1-acetoxy-2,3,5-tribenzoyloxy-1-beta-D-ribofuranose, which has the advantages of simple method, low environmental pollution, low preparation cost, The product has the advantages of high quality and is suitable for industrial application.
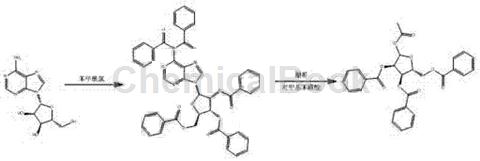
(1) Benzoylation reaction: Add 53.4g adenosine and 110g triethylamine to 500mL methylene chloride, heat to dissolve, cool to 5-10°C in an ice water bath, add 160g benzoyl chloride dropwise, Control the reaction temperature to not exceed 15°C, and complete the dropwise addition in 30 minutes. After the dropwise addition, the temperature is raised to 20-30°C and the reaction is completed in 12h. Suction filtration, wash the filter cake with an appropriate amount of methylene chloride, combine the filtrate, concentrate to dryness under reduced pressure to recover the solvent, add methanol to the remaining residue, stir and beat, filter to obtain crude product, dry in a blast drying oven to obtain 145g of white benzoyl material .
(2) Acetylation reaction: Add the above 145g benzoyl compound to 1.45L methylene chloride, stir to dissolve, add 54g acetic anhydride, cool to 10-20°C in an ice water bath, add p-toluenesulfonic acid 2g, control the reaction temperature not to exceed 20°C, and continue the reaction at this temperature for 12 hours. Suction filtration, wash the filter cake with an appropriate amount of methylene chloride, combine the filtrate, wash the filtrate with 500mL water, 500mL saturated sodium bicarbonate solution and 500mL saturated brine in sequence, dry over anhydrous magnesium sulfate, filter, concentrate under reduced pressure to recover the solvent, and obtain a white color The crude product is recrystallized with ethanol to obtain the product, which is dried in a blast drying oven to obtain white crystalline form of 1-acetoxy-2,3,5-tribenzoyloxy-1-beta-D-ribofuranose. 71.5g of powder, yield 70.9%, purity 99.1% (HPLC), melting point 129-131°C, specific rotation 42.444° (c=1, chloroform).
1HNMR (400MHz, CDCl3) δ: 2.003 (s, 3H, CH3COO), 4.484-4.531 (m, 1H, H-5), 4.767-4.817 (m, 2H, H-4andH-5), 5.786- 5.930 (m, 2H, H-2andH-3), 6.431 (s, 1H, H-1), 7.325-8.094 (m, 15H, Ar-H).
Main reference materials
[1] CN201810789462.4 A method for preparing 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose

 微信扫一扫打赏
微信扫一扫打赏

