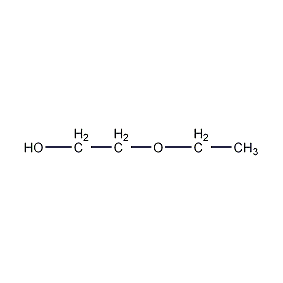
structural formula
| business number | 0324 |
|---|---|
| molecular formula | c4h10o2 |
| molecular weight | 90.12 |
| label |
ethylene glycol monoethyl ether, ethylene glycol monoethyl ether, ethyl cellosolve, 2-ethoxyethanol, ethylene glycol ether, ethylene glycol ether, ethyl cellosolve, ethylene glycol monoethylether, cellosolve, emulsion stabilizer, paint thinner, jet combustion additives, leather colorants, car engine detergent |
numbering system
cas number:110-80-5
mdl number:mfcd00002869
einecs number:203-804-1
rtecs number:kk8050000
brn number:1098271
pubchem number:24847824
physical property data
1. properties: colorless oily liquid with ether smell. [1]
2. melting point (℃): -70[2]
3. boiling point (℃): 135.1[3]
4. relative density (water=1): 0.94 (15℃)[4]
5. relative vapor density (air = 1): 3.10[5]
6. saturated vapor pressure (kpa): 0.51 (20℃)[6]
7. critical pressure (mpa): 4.24[7]
8. octanol/water partition coefficient: -0.32 [8]
9. flash point (℃): 43 (cc); 49 (oc) [9]
10. ignition temperature (℃): 235[10]
11. explosion limit (%): 15.6[11]
12. lower explosion limit (%): 1.7[12]
13. solubility: miscible with water, miscible with most organic solvents such as ethanol, ether, acetone, etc. . [13]
14. viscosity (mpa·s, 20ºc): 2.05
15. viscosity (mpa·s, 25ºc): 1.85
16. heat of evaporation (kj/mol, b.p.): 40.56
17. heat of evaporation (kj/mol, 25ºc): 47.28
18. specific heat capacity (kj / (kg·k), 25ºc, constant pressure): 2.32
19. conductivity (s/m, 20ºc): 9.3×10-8
20. vapor pressure (kpa, 25ºc): 0.7
21. volume expansion coefficient (k-1): 0.00097
22. solubility parameter (j·cm-3)0.5: 21.541
23.van der waals area (cm2·mol -1): 8.230×109
24. van der waals volume (cm3·mol -1): 56.100
25. liquid phase standard hot melt (j·mol-1·k-1): 211.1
toxicological data
1. acute toxicity[14]
ld50: 2125mg/kg (rat oral); 3900mg/kg (rat oral skin); 3300mg/kg (rabbit transdermal)
lc50: 2000ppm (rat inhalation, 7h)
2. irritation[15]
home rabbit transdermal: 500 mg, mild stimulation (open stimulation test).
rabbit eye: 50mg, moderate irritation.
3. subacute and chronic toxicity[16] rats were exposed to 1.49g/m3, 7 hours a day , 5 days a week for 5 weeks, has a slight effect on blood cell components. rabbits were given oral administration of 0.1ml/kg per day. temporary proteinuria and hematuria occurred on the 7th day; 1ml/kg resulted in death due to renal damage on the 8th day.
4. mutagenicity [17] sperm morphology: rat oral administration: 23400mg/kg, 5 weeks (intermittent). sister chromatid exchange: hamster ovary 3170mg/l.
5. teratogenicity [18] the lowest toxic dose (tdlo) of 600 mg/kg was administered orally to rats on 10 to 12 days after pregnancy, causing developmental malformations of the musculoskeletal system and cardiovascular system. the lowest toxic dose (tdlo) of 50g/kg when exposed to the skin of rats 7 to 16 days after pregnancy can cause developmental malformations in the central nervous system and genitourinary system.
6. others[19] the lowest oral toxic dose in rats (tdlo): 600mg/kg (gestation 10~12d), causing embryonic damage toxicity (such as embryonic development retardation), causing abnormal development of skeletal muscles and cardiovascular (circulatory) system. the lowest oral toxic dose in mice (tdlo): 25g/kg (25 days, male), affecting the testicles, epididymis and vas deferens.
ecological data
1. ecotoxicity[20]
lc50: >10000mg/l (96h) (bluegill sunfish); >5000mg/ l (24h) (daphnia)
2. biodegradability[21]
aerobic biodegradability ( h): 168~672
anaerobic biodegradability (h): 672~2688
3. non-biodegradability[22]
in the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 1d ( theory).
molecular structure data
1. molar refractive index: 23.86
2. molar volume (cm3/mol): 98.4
3. isotonic specific volume (90.2k ): 227.6
4. surface tension (dyne/cm): 28.6
5. polarizability (10-24cm3): 9.45
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 29.5
7. number of heavy atoms: 6
8. surface charge: 0
9. complexity: 21.5
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. stability[23] stable
2. incompatible substances[24] strong oxidants, acids, alkalis
3. conditions to avoid contact[25] air, light
4. hazards of aggregation[26] no aggregation
storage method
storage precautions[27] stored in a cool, ventilated warehouse. keep away from fire and heat sources. the storage temperature should not exceed 37°c. the packaging must be sealed and must not come into contact with air. they should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. use explosion-proof lighting and ventilation facilities. it is prohibited to use mechanical equipment and tools that are prone to sparks. the storage area should be equipped with emergency release equipment and suitable containment materials.
synthesis method
1. obtained from the reaction of ethylene oxide and ethanol. at 25-30°c, slowly add ethylene oxide to absolute ethanol, wait until the temperature rises to 70°c, and leave overnight after the reaction is completed. recover the ethanol, neutralize it with 10% sodium hydroxide solution to ph=8, and fractionate to obtain the crude product. distillate and collect the 133.5-135.5°c fraction to obtain the finished product. each ton of product consumes 863kg of ethylene oxide and 1020kg of anhydrous ethanol.
![]()
refining method: easily contained impurities there is moisture, diethylene glycol ether (ethyl carbitol), ethylene glycol and trace amounts of acids, aldehydes, etc. during refining, add calcium chloride or anhydrous sodium sulfate to dry, and then fractionate. if peroxide is present, it can be removed by refluxing tin dichloride or passing it through an activated alumina column.
purpose
1. this product is commonly used as a solvent for nitrocellulose, cellulose acetate, synthetic resin, and paint; in the leather industry, it is used as a colorant, emulsion stabilizer, and ink solvent; in the paint industry, it is used as a refined solvent for vitamin b12; in the paint industry, it is used to prepare paint thinners , paint strippers and raw materials for making spray paints; dyeing agents used in the textile industry to make fibers; used in the organic chemical industry to make acetates, emulsion stabilizers, etc. in addition to the above applications, this product can also be used as a solvent for wood coloring dyes, a solvent for petroleum soap and petroleum sulfonic acid, an intermediate for non-paint colorants in the organic synthesis industry, and an intermediate for the preparation of organic compounds, as well as analytical chemical reagents, etc. . polyethylene glycol monoethyl ether is mainly used as a high boiling point solvent for coatings, inks, dyes, resins, and nitrocellulose. in addition, this product is also widely used in the formulation of advanced automotive brake fluids and can also be used as an intermediate for the preparation of ester derivatives. body etc.
2. general solvents and solvents for the electronic industry. used in leather colorants, emulsions, stabilizers, etc.
3. a solvent with excellent performance. used as a solvent for nitrocellulose, resins, paints, oil-soluble dyes and enamels. it is also used as pesticide dispersant, fiber wetting agent, pharmaceutical extractant, fabric and plastic stain remover, metal cleaner, etc.
4. used as solvent, leather colorant, emulsifier, stabilizer, paint thinner, paint remover, etc. [28]
prepare intermediates of organic compounds, as well as analytical chemical reagents, etc. polyethylene glycol monoethyl ether is mainly used as a high boiling point solvent for coatings, inks, dyes, resins, and nitrocellulose. in addition, this product is also widely used in the formulation of advanced automotive brake fluids and can also be used as an intermediate for the preparation of ester derivatives. body etc.
2. general solvents and solvents for the electronic industry. used in leather colorants, emulsions, stabilizers, etc.
3. a solvent with excellent performance. used as a solvent for nitrocellulose, resins, paints, oil-soluble dyes and enamels. it is also used as pesticide dispersant, fiber wetting agent, pharmaceutical extractant, fabric and plastic stain remover, metal cleaner, etc.
4. used as solvent, leather colorant, emulsifier, stabilizer, paint thinner, paint remover, etc. [28]

 微信扫一扫打赏
微信扫一扫打赏

