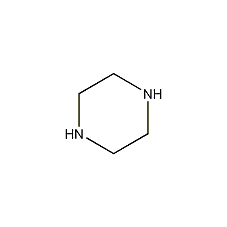
Structural formula
| Business number | 0328 |
|---|---|
| Molecular formula | C4H10N2 |
| Molecular weight | 86.14 |
| label |
None |
Numbering system
CAS number:110-85-0
MDL number:MFCD00005953
EINECS number:203-808-3
RTECS number:TK7800000
BRN number:102555
PubChem number:24887569
Physical property data
1. Character: colorless crystal
2. Density (g/mL, 20℃): 1.1
3. Relative vapor density (g/mL, air=1 ): Undetermined
4. Melting point (ºC): 106 (anhydrous), 44 (hexahydrate)
5. Boiling point (ºC, normal pressure): 146 ( Anhydrate), 125~130 (hexahydrate)
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 1.4460
8. Flash point (ºC): 65
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20ºC): 0.8
12. Saturated vapor pressure (kPa, 111ºC): 30.3
13. Heat of combustion (KJ /mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water Log value of the (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V /V): Undetermined
19. Solubility: Easily soluble in water, soluble in alcohol, insoluble in ether.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2050mg/kg
Mouse oral LD50: 600mg/kg
Rabbit dermal LD50: 4000mg/kg
p>
Mouse inhalation LC50: 5400mg/m3/2H
2. Irritation: Transdermal in rabbits, severe irritation. Rabbit eye: 250ug/24H severe irritation.
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies. It is harmful to fish and plankton, and is also harmful to organic matter in the water.
Molecular structure data
1. Molar refractive index: 25.14
2. Molar volume (cm3/mol): 98.5
3. Isotonic specific volume (90.2K ): 225.8
4. Surface tension (dyne/cm): 27.5
5. Polarizability (10-24cm3): 9.96
Compute chemical data
1. Hydrophobic parameter calculation��Reference value (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 24.1
7. Number of heavy atoms :6
8. Surface charge: 0
9. Complexity: 26.5
10. Number of isotope atoms: 0
11 .Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with moist air and direct sunlight. It is prohibited to come into contact with strong oxidants, strong acids, acid chlorides, and acid anhydrides.
Storage method
Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Piperazine hydrochloride is synthesized from chloroethanol through ammoniation and cyclization, and then neutralized with sodium hydroxide to prepare piperazine hexahydrate.
2. Using chloroethanol as raw material, it is obtained through ammoniation and cyclization. The process is to heat chloroethanol and ammonium hydroxide in an autoclave until the ammonia pressure reaches 441 kPa, stir and react for 5 hours, and recover excess ammonium hydroxide under normal pressure to obtain an aminoethanol aqueous solution. Add a certain amount of hydrochloric acid and stir, concentrate under reduced pressure into a semi-pasty ethanolamine hydrochloride concentrate, heat up to 150°C to remove water, add previously melted paraffin, continue to heat up to 260°C, and maintain (260±2)°C for 12 hours. , filter the material, remove the paraffin, and obtain piperazine hydrochloride. Add piperazine hydrochloride, solid sodium hydroxide, and water into the reaction kettle, stir for 1.5 hours, discharge ammonia, then raise the temperature to 130~140°C, steam out the piperazine hexahydrate solution, filter the residue, and freeze the piperazine hexahydrate solution to 8°C, centrifuge and filter to obtain piperazine hexahydrate, and dehydrate piperazine hexahydrate to obtain the finished product of piperazine. The reaction equation is: ClCH2CH2OH+NH4OH→NH2CH2CH2OH·HCl+H2O
Purpose
Important pharmaceutical intermediates, which can be used to synthesize piperazine phosphate, piperazine citrate, piperazine adipate, guanidine piperazine, methyltetracycline, quinipamate, penitiazole, enoxacin, hydroxyl hydrochloride Chlorazine, perphenazine, trifluoperazine, diethylcarbamazine citrate, cinnarizine, flunarizine, dechlorhydrizine, tetrazine, fluphenazine, prednisone sodium phosphate, dexamethasone Medications such as metasone sodium phosphate, piperonic acid, norfloxacin, ciprofloxacin, ethidium, meperimycin, and trimethoprim. It is also used in the production of wetting agents, emulsifiers, dispersants, antioxidants, preservatives, stabilizers, plastic processing and rubber additives, etc.

 微信扫一扫打赏
微信扫一扫打赏

