Background and overview[1][2]
Di-p-tolylmethane is a compound that can be used as a high-boiling organic solvent.
Preparation[2]
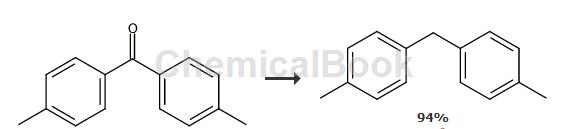
General/Typical Procedure: To a solution of diaryl ketone (10.0 mmol) in methanol (20 mL) was added Pd/C (20%, 200 mg). The reaction mixture was stirred at room temperature under a hydrogen atmosphere for 16 hours. The solvent was removed under reduced pressure and the product was purified by flash column chromatography on silica gel using pentane as eluent. For di-p-tolylmethane, refer to the above method, yield: 94%, white solid. 1H NMR (600MHz, CDCl3) δ (ppm) 7.02-6.99 (m, 8H), 3.83 (s, 2H), 2.23 (s, 6H). 13CH NMR (150MHz, CDCl3) δ (ppm) 138.3, 135.4, 129.1, 128.7, 41.1, 21.0. MS (EI): m/z = 196 (M+, 77), 181 (100), 105 (9), 77 (10).
Apply[1]
A method for preparing 2,4-diaminobenzenesulfonic acid, which method includes the following steps: reacting m-phenylenediamine and sulfuric acid or fuming sulfuric acid in a solvent in a temperature range of 140 to 250°C, To prepare 2,4-diaminobenzenesulfonic acid, the solvent is one or more of the following solvents: an inorganic solvent selected from phosphoric acid or polyphosphoric acid and an organic solvent with a normal pressure boiling point above 140°C. The organic solvent is one or more selected from the following solvents: xylene, trimethylbenzene, cumene, isobutylbenzene, tert-butylbenzene, cumene, naphthalene, methylnaphthalene, Tetralin, diphenylmethane, di-p-tolylmethane, dimethylchlorobenzene, trimethylchlorobenzene, dichlorobenzene, trichlorobenzene, bromobenzene, chlorotoluene, dichlorotoluene, bromotoluene, xylene Bromobenzene, nitrobenzene, nitrotoluene, nitroethylbenzene, nitroxylene, trimethylnitrobenzene, nitrochlorobenzene, acetophenone, nonane, undecane, paraffin, chlorinated paraffin , high temperature kerosene, thermal oil, tetrabromoethane, trichloropropane, tetrabromobutane, cyclohexanone, sulfolane, succinonitrile and adiponitrile.
Main reference materials
[1] [Chinese invention, Chinese invention authorization] CN200610029051.2 Synthesis method of 2,4-diaminobenzenesulfonic acid and its salt
[2] Cheng Y , Dong W , Wang L , et al. Iron-Catalyzed Hetero-Cross-Dehydrogenative Coupling Reactions of Sulfoximines with Diarylmethanes: A New Route to N-Alkylated Sulfoximines[J]. Organic Letters, 2015, 45(37):2000.

 微信扫一扫打赏
微信扫一扫打赏

