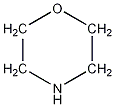
Structural formula
| Business number | 032D |
|---|---|
| Molecular formula | C4H9NO |
| Molecular weight | 87.12 |
| label |
Tetrahydro-1,4-oxazine, 1,4-Oxazacyclohexane, 1,4-Oxaazepine, Tetrahydro-1,4-oxazine, Morpholine, morpholine, 1,4-oxazacyclohexane, p-oxazine, 1-Oxa-4-azacyclohexane, 1,4-Oxazinan;Tetrahydro-1,4-Oxazinan, heterocyclic compounds, Corrosion inhibitor |
Numbering system
CAS number:110-91-8
MDL number:MFCD00005972
EINECS number:203-815-1
RTECS number:QD6475000
BRN number:102549
PubChem number:24864597
Physical property data
1. Properties: colorless oily hygroscopic liquid with ammonia smell. [11]
2. Melting point (℃): -5[12]
3. Boiling point (℃): 128.9[13]
4. Relative density (water = 1): 1.00[14]
5. Relative vapor Density (air=1): 3.0[15]
6. Saturated vapor pressure (kPa): 1.06 (20℃)[16]
7. Critical temperature (℃): 344[17]
8. Critical pressure (MPa): 5.302[18]
9. Octanol/water partition coefficient: -0.86[19]
10. Flash point (℃): 38 (OC)[20]
11. Ignition temperature (℃): 310[21]
12. Explosion upper limit (%): 11.2[22]
13. Lower explosion limit (%): 1.4[23]
14. Solubility: with It is miscible in water and can be miscible in most organic solvents. [24]
15. Viscosity (mPa·s, 15ºC): 2.5334
16. Viscosity (mPa·s, 20ºC): 2.33
17. Heat of evaporation (KJ/mol): 43.96
18. Heat of fusion (KJ/mol): 14.53
Toxicological data
1. Acute toxicity[25]
LD50: 1450mg/kg (rat oral); 525mg/kg (orally for mice); 500μl (500mg)/kg (for rabbits through dermally)
LC50: 8000ppm (rat inhalation, 8h)
2. Irritation[26]
Rabbit transdermal: 995mg (24h), severe irritation.
Rabbit eye: 2 mg, severe irritation.
3. Subacute and chronic toxicity[27] Rat inhalation 6.4g/m3 (repeated inhalation), eye and respiratory tract irritation, lung, liver and kidney lesions.
Ecological data
1. Ecotoxicity[28]
LC50: 350~400mg/L (96h) (fish )
IC50: 1.7~4.1mg/L (72h) (algae)
2Produced by reaction at �� temperature. Or it is prepared by the action of diethanolamine and excess concentrated hydrochloric acid (or concentrated sulfuric acid).
4. Morpholine can be produced by closing the ring of diethanolamine in an acidic environment, and then purifying it by distillation.
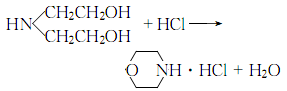
Purpose
1. Used as analytical reagents and solvents for resins, waxes, shellac, etc. It is widely used as solvent for resin, dye, wax, shellac, casein, etc., boiler preservative and rubber vulcanization accelerator. It is also used in the preparation of surfactants, plasticizers, antioxidants and medicines.
2. Used as a water treatment corrosion inhibitor. Because its aqueous solution is alkaline, it can neutralize free carbon dioxide in the water, increase the PH value of boiler feed water, and slow down the corrosion of carbon dioxide. It can prevent the corrosion of high-temperature acidic oxides in boiler feed water.
3. In medicine, it is used to synthesize many important drugs such as Viralin, ibuprofen, metoclopramide, flubiprofen, and mefenamic acid. In organic synthesis, it can be used as intermediates and raw materials for N-methylmorpholine, N-ethylmorpholine, morpholine fatty acid salts, etc.
4. This reagent can be used as a base to synthesize enamines and as a catalyst in Mannich condensation reaction.
Willgerodt reaction Morphinol can be used as the amine component in Kindler’s improved Willgerodt reaction [2], and it can also occur under microwave irradiation This rearrangement reaction (Formula 1, Formula 2)[3].
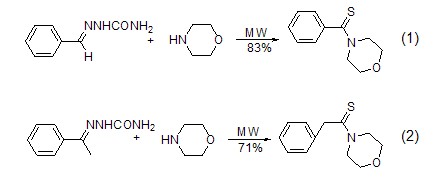
Formation of imine Morpholine is one of the three main secondary amines used in the synthesis of enamines. It is less basic than piperidine and tetrahydropyrrole, so the formation rate of enamine is slower than that of piperidine and tetrahydropyrrole. In the process of forming enamine, the regioselectivity of morpholine is also worse than that of pyridine and tetrahydropyrrole[4]. When synthesizing enamines from saturated aldehydes, ketones and morpholine, the reaction can be directly heated in benzene[5], or catalyzed by p-toluenesulfonic acid or titanium tetrachloride (Formula 3) [6], you can also use microwave irradiation[7], or react with enol trifluorosulfonate under Pd catalysis to form (formula 4)[8].
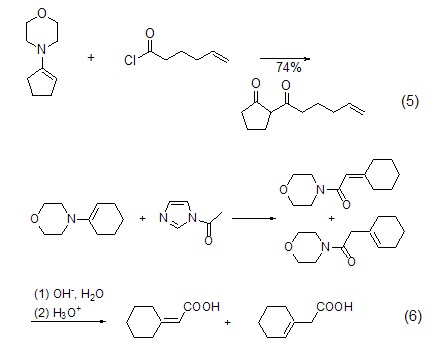
Morphine enamine Application Although morpholine is not very active when synthesizing enamines, it has a high yield when synthesizing pyrrolidone derivatives. A typical example is the acylation of cyclopentenomorpholine enamine (Formula 5)[9]. The reaction with acetylated imidazole can also form amide of morpholine enamine, and acidification is obtained after acidification (Formula 6)[10].
5. Used as analytical reagents, And solvents for resins, waxes, shellac, etc. [34]

 微信扫一扫打赏
微信扫一扫打赏

