Overview[1]
Diphenyl iodide hexafluoroarsenate can be used as a pharmaceutical synthesis intermediate. Existing studies have prepared diaryliodonium polyhalomethanes by reaction between diaryliodonium disulfates and alkali metal polyhaloformates. There is also research on using methylene chloride as a solvent instead of acetic acid in the reaction during the formation of disodium diaryl hydrogen sulfate salt. Although satisfactory results were obtained by the above method, considerable amounts of polyhalomethyl salts, such as potassium hexafluoroarsenate, were required in the diaryliodonium bisulfate reaction mixture to achieve the desired diaryliodonium Optimal yields of polyhalogenated metalloid salts. As a result, the diaryliodonium disulfate to diaryliodonium polyhalogenated nonmetal salt route is less economically attractive due to the significant loss of the polyhalomethylal salt.
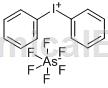
Structure
Preparation method[1]
Diphenyl iodide hexafluoroarsenate is prepared as follows: slowly add about 25 parts of concentrated sulfuric acid to a mixture of 25 parts of potassium iodate, 32 parts of benzene, 50 parts of acetic anhydride and 60 parts of methylene chloride at -10°C. middle. The mixture was stirred for 4 hours and maintained at -5°C, then slowly warmed to 25°C. The mixture is then stirred for a further 12 hours and approximately 100 parts of water are slowly added. . The lower dichloromethane layer was removed from the reaction mixture and discarded. Add 14.3 parts of sodium perchlorate to the water layer to obtain precipitation. Based on the preparation method, the precipitate is diphenyliodonium perchlorate. The diphenyliodonium perchlorate is then dried. A mixture of 2 parts diphenyliodonium perchlorate, 1.2 parts potassium hexafluoroarsenate and approximately 60 parts methyl ethyl ketone was stirred for 1 hour. The mixture was then filtered to remove precipitated potassium perchlorate. Methyl ethyl ketone was evaporated to obtain a crystalline product with a melting point of 123-125°C. According to the preparation method, the product was diphenyl iodide hexafluoroarsenate, with a yield of 96%.
Main reference materials
[1] US4329300 Method for making diaryliodoniumpolyhalometalloids

 微信扫一扫打赏
微信扫一扫打赏

