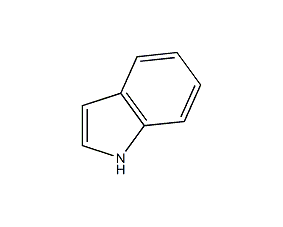
Structural formula
| Business number | 03CQ |
|---|---|
| Molecular formula | C8H7N |
| Molecular weight | 117 |
| label |
None |
Numbering system
CAS number:120-72-9
MDL number:MFCD00005607
EINECS number:204-420-7
RTECS number:NL2450000
BRN number:107693
PubChem number:24896037
Physical property data
1. Properties: colorless flaky crystals with strong fecal odor.
2. Melting point (℃): 52.5
3. Boiling point (℃): 254
4. Boiling point (℃, 3.7kpa): 128~133
5. Relative density: 1.22
6. Solubility: soluble in hot water and benzene, easily soluble in ethanol, ether and toluene.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 38.52
2. Molar volume (cm3/mol): 101.8
3. Isotonic specific volume (90.2K): 270.7
4. Surface tension (dyne/cm): 49.8
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability 15.27
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 15.8
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 101
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It can volatilize with water vapor, turn red when placed in the air or exposed to light, and become resinous. Highly dilute solutions can be used as fragrances.
Storage method
Seal and store in a dry and dark place at 4°C.
Synthesis method
1. Extraction from washed oil fractions. High-temperature coal tar contains about 0.10%-0.16% of indole. It can generally be extracted from coal tar and wash oil fractions. The washed oil fraction is washed with alkali and acid to obtain the methylnaphthalene fraction, which is then rectified in a high-efficiency tower with 60 theoretical plates. The 225-256°C fraction is cut out and melted with potassium hydroxide. The reaction was carried out at 170-240°C for 2-4 hours and stirred until bubbling stopped. Let it stand, release the lower layer of indole potassium to cool, break it into pieces, and wash it with benzene at low temperature to remove oil. Then hydrolyze at 50-70°C to obtain crude indole oil, which is distilled in a distillation tower with 20 theoretical plates. The reflux ratio is 8-10:1, and the fraction with a top temperature of 170-256°C is cut, cooled, and crystallized. , centrifugal filtration, that is��Refined indole. After pressing, the oil content is below 3%, and then recrystallized with ethanol to obtain refined indole with a purity of 99%.
2. Prepared by catalytic dehydrogenation of o-aminoethylbenzene. O-aminoethylbenzene is dehydrogenated and cyclized at 550°C in the presence of aluminum nitrate (or aluminum oxide) in a nitrogen flow, and distilled under reduced pressure to obtain indoline. Then dehydrogenate at 640°C to obtain indole. Other preparation methods include the reaction of o-nitrotoluene and oxalate to generate o-nitrophenylpyruvate, which is then made into α-indolecarboxylic acid, and finally the product is obtained by carbonization with lime; aniline and acetylene are mixed in Heating at 600-650℃ to synthesize indole; o-carboxyphenylglycine is synthesized through 3-hydroxy-2-indolecarboxylic acid and indole acid; oxidizing static blue with concentrated nitric acid or chromic acid to obtain indole quinone, and then Indole can be obtained by distillation with zinc powder. Indole can also be obtained by mixing nitrocinnamic acid and 10 parts of potassium hydroxide powder, adding iron filings and heating the mixture to melt it. For the synthesis of indole and its homologues, the Fisher synthesis method is the most common. Aromatic hydrazones of ketones or aldehydes react under acidic conditions and undergo rearrangement reactions similar to benzidine to generate indole.
Purpose
Mainly used as raw materials for spices, dyes, amino acids, and pesticides. Indole itself is also a spice, often used in daily fragrance formulas such as jasmine, lilac, lotus and orchid, and the dosage is generally a few thousandths.

 微信扫一扫打赏
微信扫一扫打赏

