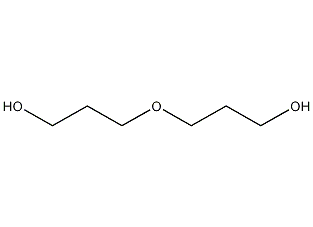
Structural formula
| Business number | 032K |
|---|---|
| Molecular formula | C6H14O3 |
| Molecular weight | 134.18 |
| label |
Dipropylene glycol chloride, 1,2-propylene glycol ether, Propylene glycol-(1,2)-ether, Dihydroxydipropyl ether, Bis(2-hydroxypropyl) ether, dipropylene glycol, Dipropylene glycol, 1,1′-Oxydi-2-propanol, 1,1′-Dimethyldiethylene glycol, fiber lubricants, plasticizer, fumigant, Synthetic detergent |
Numbering system
CAS number:110-98-5
MDL number:MFCD00004538
EINECS number:246-770-3
RTECS number:UB8785000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: colorless, odorless, slightly viscous liquid, hygroscopic.
2. Density (g/mL, 20/4℃): 1.0252
3. Relative vapor density (g/mL, air=1): 4.63
4. Melting point (ºC, pouring point): -40
5. Boiling point (ºC, normal pressure): 232
6. Refractive index (20ºC): 1.4440
p>
7. Viscosity (mPa·s, 20ºC): 107
8. Flash point (ºC, opening): 138
9. Heat of vaporization (KJ/mol ): 96.3
10. Autoignition point or ignition temperature (ºC): 310
11. Vapor pressure (kPa, 20ºC): <0.00133
12 . Vapor pressure (kPa, 114ºC): 1.33
13. Vapor pressure (kPa, 151ºC): 6.67
14. Volume expansion coefficient (K-1, 20ºC): 0.00073
15. Explosion upper limit (%, V/V): 12.7
16. Explosion lower limit (%, V/V): 2.9
17. Solubility: miscible with water. It is also miscible with oils, natural resins, nitrocellulose, aromatic hydrocarbons, etc., but is not miscible with aliphatic hydrocarbons.
Toxicological data
1. Acute toxicity: rat oral LD50: 14800mg/kg
2. It is slightly toxic, rat oral LD50 is 14.85mg/kg. The rabbit transdermal LD50 is above 20mL/kg. Adding 5% dipropylene glycol to the drinking water of rats had no effect on the animals after 77 days. If it increases to 10%, some animals will die due to edema and degeneration of renal tubular epithelial cells and liver parenchyma.
Ecological data
This substance may be harmful to the environment and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 34.66
2. Molar volume (cm3/mol): 128.9
3. Isotonic specific volume (90.2K ): 323.9
4. Surface tension (dyne/cm): 39.8
5. Polarizability (10-24cm3): 13.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.4
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
p>
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 49.7
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 57.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It does not decompose under normal temperature and pressure. Contact with strong oxidants is prohibited. It has the general chemical properties of alcohol.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Originated from the condensation of 1,2-propanediol and propylene oxide. Condensation reaction of 1,2-propanediol and propylene oxide is carried out under the action of 85% sulfuric acid. The reaction solution is neutralized with sodium hydroxide solution, filtered, distilled under reduced pressure, and then rectified under normal pressure. The 229-233°C fraction is collected to obtain dipropylene glycol. Raw material consumption quota: propylene glycol 1300kg/t, propylene oxide 1500kg/t, sulfuric acid (98%) 100kg/t, caustic soda (95%) 100kg/t.
Refining method: In addition to 1,2-propanediol and tripropylene glycol, it is easy to contain impurities, including a small amount of moisture and acidic substances. It is generally refined by vacuum distillation. Collect the middle fraction, dry it over anhydrous sodium sulfate and repeat the fractionation.
Purpose
Used in the manufacture of polyester resin. Used as a solvent for cellulose acetate, cellulose nitrate, shellac varnish, castor oil, a component of fiber lubricants, and for the preparation of plasticizers, fumigants, synthetic detergents, etc. In addition, dipropylene glycol can be used alone or mixed with diethylene glycol to extract aromatics from hydrocarbons.

 微信扫一扫打赏
微信扫一扫打赏

