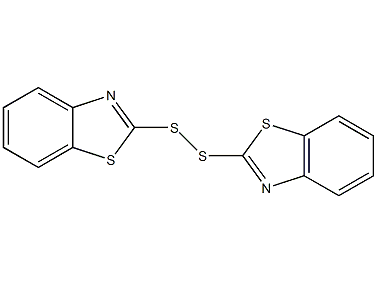
Structural formula
| Business number | 03CU |
|---|---|
| Molecular formula | C14H8N2S4 |
| Molecular weight | 332.49 |
| label |
accelerator DM, accelerator MBTS, Rubber accelerator DM, Vulcanization accelerator DM, Dibenzothiazole disulfide, Accelerator DM, Accelerator MBTS, Rubber accelerator DM, Vulcanization accelerator DM, Diphenyl disulfide, benzothiazole, vulcanization activator |
Numbering system
CAS number:120-78-5
MDL number:MFCD00022874
EINECS number:204-424-9
RTECS number:DL4550000
BRN number:None
PubChem number:24893650
Physical property data
1. Character: light yellow needle-like crystal
2. Relative density (20/4℃): 145-1.50
3. Flash point (℃): 271
4. Melting point (℃): 213-220
5. Water solubility (g/100mL, 20 ºC): <0.01
Toxicological data
Toxicity classification Poisoning
Acute toxicity Abdominal cavity-rat LD50: 2600 mg/kg; abdominal cavity-mouse LD50: 100 mg/kg
This product is toxic in contact with skin Irritating.
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 94.48
2. Molar volume (cm3/mol): 211.7
3. Isotonic specific volume (90.2K ): 652.8
4. Surface tension (dyne/cm): 90.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability 37.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 5.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 133
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 311
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of stereocenters of chemical bondsAmount: 0
15. Number of covalent bond units: 1
Properties and stability
Light yellow needle-like crystals precipitate from benzene. Insoluble in water, ethyl acetate, gasoline and alkali. Solubility (g/100ml) in the following substances at 25°C is: ethanol <0.2, acetone <0.5, benzene <0.5, carbon tetrachloride <0.2, ether <0.2 . Slightly bitter and odorless. Slightly soluble in benzene, toluene, carbon tetrachloride, dichloromethane, acetone, ethanol, ether, etc. at room temperature, with very little toxicity. Can burn in case of open flame. There is a risk of explosion in the form of dust.
Storage method
Keep away from high heat sources. Granular or powdery products have a storage stability period of more than two years and must be stored according to regulations for toxic substances.
Be careful to prevent moisture.
Synthesis method
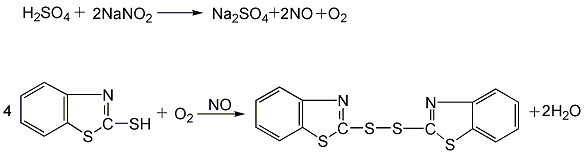
Add 2-mercaptobenzothiazole (see accelerator M) and sodium nitrite into the gas phase bubble tower, add 10% sulfuric acid solution dropwise, and bubble in air. Control the reaction temperature at about 60°C. After the sulfuric acid is added dropwise, air is continued to be blown in, and the temperature is maintained at 60°C until the reaction is complete.
The generated accelerator DM is insoluble in water and appears as solid particles in the feed liquid. After filtering, washing, centrifugal dehydration, drying, crushing, screening and packaging, the finished product is obtained.
Purpose
1. Thiazole vulcanization accelerator. It can be used in natural rubber, synthetic rubber and reclaimed rubber. Its uses are basically similar to accelerator M, but the critical vulcanization temperature is higher (130°C). It is one of the commonly used vulcanization accelerators and is widely used in rubber products such as tires, hoses, rubber mats, tarpaulins, brightly colored rubber products, hoses, wires, cables, rubber shoes and other non-food applications. This product has low toxicity, but can irritate mucous membranes and skin
2. This product is used as an active agent during sulfur vulcanization of unsaturated polyurethane rubber. .

 微信扫一扫打赏
微信扫一扫打赏

