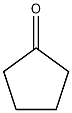
Structural formula
| Business number | 03CZ |
|---|---|
| Molecular formula | C5H8O |
| Molecular weight | 84.12 |
| label |
Adipic keyone, Ketocyclopentane, Ketopentamethylene, spices, Alicyclic compounds |
Numbering system
CAS number:120-92-3
MDL number:MFCD00001409
EINECS number:204-435-9
RTECS number:GY4725000
BRN number:605573
PubChem number:24846157
Physical property data
1. Properties: water-white liquid with an ether-like odor. [1]
2. Melting point (℃): -51.3[2]
3. Boiling point (℃): 130.6[3]
4. Relative density (water = 1): 0.95[4]
5. Relative vapor Density (air=1): 2.3[5]
6. Saturated vapor pressure (kPa): 1.52 (25℃)[6]
7. Critical pressure (MPa): 5.2[7]
8. Octanol/water partition coefficient: 0.63[8]
9. Flash point (℃): 26; 30.5 (CC) [9]
10. Explosion limit (%): 10.4[10]
11. Lower explosion limit (%): 1.7[11]
12. Solubility: insoluble in water , soluble in most organic solvents such as ethanol, ether, acetone and so on. [12]
13. Eccentricity factor: 0.388
14. Solubility parameter (J·cm-3)0.5: 21.103
15. van der Waals area (cm2·mol-1): 7.000×109
16. van der Waals volume (cm3·mol-1): 52.620
17. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2875.2
18. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -235.7
19. Liquid phase standard hot melt (J·mol-1·K-1) : 164.2
20. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2917.9
21. Gas phase standard claimed heat (enthalpy) )( kJ·mol-1): -192.1
22. Gas phase standard entropy (J·mol-1·K-1 ): 313.68
23. Gas phase standard free energy of formation (kJ·mol-1): -90.6
24. Gas phase standard heat Melting (J·mol-1·K-1): 95.33
Toxicological data
1. Acute toxicity[13]
LD50: 1820mg/kg (mouse oral); 1950mg/kg (mouse intravenous)
LC50: 19500mg/m3 (rat inhalation)
2. Irritation [14]
Rabbit transdermal: 500mg, mild stimulation.
Rabbit eye: 100mg, severe irritation.
Ecological data
1. Ecotoxicity[15] IC50: 63~1900mg/L (72h) (algae)
2. Biodegradability No data yet
3. Non-biodegradability[16] In the air, when the hydroxyl radical concentration is 5.00×1At 05 pieces/cm3, the degradation half-life is 5.5d (theoretical).
Molecular structure data
1. Molar refractive index: 23.18
2. Molar volume (cm3/mol): 85.2
3. Isotonic specific volume (90.2K ): 205.7
4. Surface tension (dyne/cm): 34.0
5. Dielectric constant: None available
6. Polarizability: 9.19
7. Single isotope mass: 84.057515 Da
8. Nominal mass: 84 Da
9. Average mass: 84.1164 Da
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 58.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[17] Stable
2. Incompatible substances [18] Strong oxidizing agent, strong alkali, strong reducing agent
3. Polymerization hazard[19] No aggregation
Storage method
Storage Precautions[20] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained by heating adipic acid in the presence of barium hydroxide. Mix adipic acid and barium hydroxide evenly, heat to 285-295°C, and distill out the generated cyclopentanone at this temperature. The distillate is salted out with calcium chloride to separate cyclopentanone. Add a certain amount of alkali solution to remove adipic acid, then wash with water. After drying with anhydrous calcium chloride, fractionate and collect the 128-131°C fraction to obtain the finished product. Yield 75-80%.
2. Preparation method:
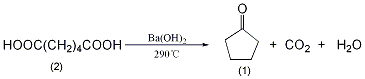
In a reaction bottle equipped with a distillation device and a thermometer (extending close to the bottom of the reaction bottle), add 200g (1.34mol) of adipic acid (2) and 10g of finely ground barium hydroxide powder, and mix thoroughly Evenly. Slowly heat over low heat to 285~290℃, and liquid will slowly come out. Maintain this temperature until only a small amount of residue remains in the reaction flask. The distillate was saturated with calcium chloride, the organic layer was separated, washed with dilute alkali and saturated brine in sequence, dried over anhydrous magnesium sulfate, fractionated, and the fractions at 128-132°C were collected to obtain 90g of cyclopentanone (1), collected The rate is 78%. [22]
Purpose
1. This product is an organic chemical raw material. It is a raw material for the pharmaceutical and spice industries. It can be used to prepare new spices, methyl hydrojasmonate, and is also used in rubber synthesis; biochemical research and as a pesticide. Used as solvents, medicines, spices, pesticides, and synthetic rubber intermediates.
2. Used as an intermediate for pharmaceuticals, biological products, pesticides and synthetic rubber. [21]

 微信扫一扫打赏
微信扫一扫打赏

