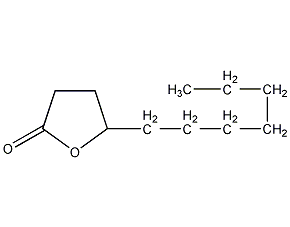
Structural formula
| Business number | 02QF |
|---|---|
| Molecular formula | C11H20O2 |
| Molecular weight | 184.28 |
| label |
Undecanoin lactone, peach aldehyde, Tetradecanal (customary commercial name), 1,4-Hendanolide, 4-Hydroxyunddecanoic acid lactone, 5-Heptyldihydro-2(3H)-furanone, artificial flavors |
Numbering system
CAS number:104-67-6
MDL number:MFCD00005405
EINECS number:203-225-4
RTECS number:YQ2485000
BRN number:81943
PubChem ID:None
Physical property data
1. Properties: Colorless to light yellow oily liquid with peach and almond aroma.
2. Density (g/mL, 25℃): 0.944
3. Relative density (20℃, 4℃): 0.8315d
4. Melting point (ºC): 25
5. Boiling point (ºC, normal pressure): 286
6. Boiling point (ºC, 1.73KPa): 136
7. Refractive index (n/20D): 1.45
8. Flash point (ºC): 137
9. Relative density (25℃, 4℃ ): 0.8278
10. Refractive index at room temperature (n25): 1.4372
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC) : Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Dissolved in Ethanol (1ml dissolved in 5ml 60% ethanol), benzyl alcohol, benzyl benzoate, propylene glycol, lye, most non-volatile oils and mineral oil, are almost insoluble in glycerin and water.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: skin contact on rabbits, 100mg/24Hreaction severity, strong reaction; Standard Dresser test: skin contact on guinea pigs, 100mg/24Hreaction severity, moderate reaction. 2. Acute toxicity: Rat oral LD50: 18500mg/kg 3. Mutagenicity: DNA repair test: Bacillus subtilis, 10mg/disc
Ecological data
None
Molecular structure data
1. Molar refractive index: 52.67
2. Molar volume (cm3/mol): 196.3
3. Isotonic specific volume (90.2K ) 464.5
4. Surface tension (dyne/cm): 31.3
5. Dielectric constant:
6. Dipole moment (10 -24cm3):
7. Polarizability: 20.88
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: 2
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 154
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Soluble in ethanol (1ml dissolved in 5mL of 60% ethanol), benzyl alcohol, benzyl benzoate, propylene glycol, alkali, most non-volatile oils and mineral oil, almost insoluble in glycerol and water.
2. Natural products are found in apples, butter, rice, peaches, apricots, osmanthus, hydrolyzed soy protein, cream, plumeria flowers, etc.
3. Exist in tobacco leaves and smoke.
Storage method
Glass bottle packaging. Store in a cool and dry place.
Synthesis method
1. The main preparation method is through the rearrangement of ω-undecenoic acid under acidic conditions to generate β-undecenoic acid, and then lactonization.
Add ω-undecenoic acid and 1.15 times the mass of 80% H2SO4 into the reaction kettle, stir and react at 80-85°C for 4 hours. Add water and stir for 0.5h, then let it stand for layering. The oil phase is washed with water, 15% Na2CO3 solution and water in sequence, dried and distilled. The fraction at 160℃~170℃ (1.73kPa) is collected as the product.
2.Esterification of undecylenic acid in the presence of sulfuric acid, followed by separation, washing and distillation.
3.Prepared from n-octanol and acrylic acid.
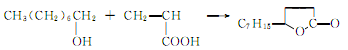
4.Using undecylenic acid obtained by pyrolysis of castor oil as raw material, it is produced through transfer and lactonization reaction.
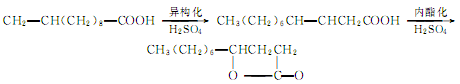
Purpose
1. Prepare peach, apricot and other food flavors; prepare osmanthus, jasmine, violet and other floral daily cosmetic flavors. Product quality: content ≥97%.
2.Used to prepare peach, cherry, coconut, apricot, plum and other flavored food flavors. The usage amount in chewing gum is 90mg/kg; in candy 11mg/kg; 7.5 mg/kg in puddings; 7.1 mg/kg in baked goods span>; 3.0mg/kg in cold drinks; 4.4mg/kg in soft drinks .
3.γ-Undecanolide is one of the most commonly used lactone flavors. It is commonly used in daily fragrances such as osmanthus, jasmine, gardenia, lily of the valley, orange blossom, white rose, lilac, and acacia. It is also a good raw material for preparing food flavors such as peaches, melons, plums, apricots, cherries, and osmanthus.

 微信扫一扫打赏
微信扫一扫打赏

