Structural formula
| Business number | 02QR |
|---|---|
| Molecular formula | C7H6Cl2 |
| Molecular weight | 161 |
| label |
1-Chloro-4-chlorotoluene, α,4-Dichlorotoluene, p-chlorobenzyl chloride, p-chlorobenzyl chloride, 4-Chlorobenzyl chloride, 4-Chlorobenzyl chloride, (4-Chlorophenyl)methylchloride, 4-Dichlorotoluene, 1-Chloro-4-(chloromethyl)-benzen, Aromatic halogen derivatives |
Numbering system
CAS number:104-83-6
MDL number:MFCD00000914
EINECS number:203-242-7
RTECS number:XT0720000
BRN number:471558
PubChem number:24854308
Physical property data
1. Properties: Colorless liquid or white needle-like crystals.
2. Density (g/mL, 20℃): 1.275
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 29
5. Boiling point (ºC, normal pressure): 222
6. Boiling point (ºC, 2.66kPa): 117
7. Refractive index: Undetermined
8. Flash point (ºC): 107
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): 610
11. Vapor pressure (mmHg, 20.2ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC) : Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): 8.6
18. Lower explosion limit (%, V/V): 2
19. Solubility: Soluble in ether, acetic acid, carbon disulfide and benzene, easily soluble in cold ethanol, can Soluble in ethanol, ether and acetone, insoluble in water.
Toxicological data
1. Acute toxicity: unknown LD50 in rats: 1075mg/kg; unknown LD50 in mice: 1156mg/kg; unknown LD50 in guinea pigs: 5625mg/kg; 2. Other multi-dose toxicity: oral TDLo in rats: 10370mg/kg /9W-I;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 40.91
2. Molar volume (cm3/mol): 129.0
3. Isotonic specific volume (90.2K): 318.3
4. Surface tension (dyne/cm): 37.0
5. Dielectric constant: 2.27
6. Dipole moment (10-24cm3):
7. Polarizability: 16.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 75
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with alkalis, water, and oxidants.
2.This product is toxic, irritating to the eyes and mucous membranes, and can induce tears. Avoid direct contact with the human body during operation, and the equipment must be sealed. Operators should wear protective equipment.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire, heat and water sources. They should be stored separately from oxidants, alkalis, etc. and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Pack airtightly in plastic buckets. Store in a cool, ventilated and dry place. Store and transport according to regulations on toxic substances.
Synthesis method
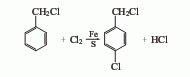
1.Benzyl chloride low temperature chlorination method The chlorination reaction is carried out in the presence of sulfur and iron powder. The ratio of sulfur to iron powder is 9: 1, and the reaction temperature is 15-17℃, when the relative density of the reaction solution increases to 1.22, the chlorination is completed. The crude product was distilled under reduced pressure to obtain the finished product, with a yield (based on benzyl chloride) 80.8% and a content 94.4% span>.
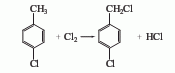
2.High-temperature chlorination method of p-chlorotoluene. First, put p-chlorotoluene into the reactor. 100℃ Start slowly passing chlorine and chlorination, and then the temperature Up to115℃The obtained crude p-chlorobenzyl chloride is washed with water, distilled under reduced pressure, and collected110-120℃(97.325kPa) fraction is the finished product.
3. Obtained from the reaction of chlorobenzene with hydrogen chloride, paraformaldehyde, etc. Mix chlorobenzene, paraformaldehyde, phosphoric acid, and zinc chloride, and introduce hydrogen chloride to react. The temperature is40-45℃ , the reaction time is about 2h. Then it is extracted with benzene, neutralized with sodium carbonate, dried and purified to obtain the finished product. In addition, p-chlorotoluene is obtained by light chlorination under the catalysis of azobisisobutyronitrile.
4. Preparation method:
 In a container equipped with a thermometer and a reflux condenser (connected to the top Calcium chloride drying tube, the drying tube is connected to the reaction bottle of hydrogen chloride and chlorine absorption device) and ventilation tube, add 100g (0.79mol) of p-chlorotoluene (2) and 0.25g of azobisisobutyronitrile, and heat to reflux. Pour in the chlorine gas while stirring. The chlorine gas can be introduced slowly at the beginning, then the chlorine gas can be introduced faster, and it can be slower when approaching the end. After induction, cool down, and steam out unreacted chlorine and generated hydrogen chloride under reduced pressure. Add anhydrous sodium carbonate powder to neutralize the acid in the reaction system until no carbon dioxide gas escapes. Filter and distill under reduced pressure to remove low boiling matter to obtain p-chlorobenzyl chloride (1) with a yield of 90%. [1]
In a container equipped with a thermometer and a reflux condenser (connected to the top Calcium chloride drying tube, the drying tube is connected to the reaction bottle of hydrogen chloride and chlorine absorption device) and ventilation tube, add 100g (0.79mol) of p-chlorotoluene (2) and 0.25g of azobisisobutyronitrile, and heat to reflux. Pour in the chlorine gas while stirring. The chlorine gas can be introduced slowly at the beginning, then the chlorine gas can be introduced faster, and it can be slower when approaching the end. After induction, cool down, and steam out unreacted chlorine and generated hydrogen chloride under reduced pressure. Add anhydrous sodium carbonate powder to neutralize the acid in the reaction system until no carbon dioxide gas escapes. Filter and distill under reduced pressure to remove low boiling matter to obtain p-chlorobenzyl chloride (1) with a yield of 90%. [1]
Purpose
It can be used in organic synthesis intermediates, pharmaceutical industry, and dye industry.
The boiling material was boiled to obtain p-chlorobenzyl chloride (1), with a yield of 90%. [1]
Purpose
It can be used in organic synthesis intermediates, pharmaceutical industry, and dye industry.

 微信扫一扫打赏
微信扫一扫打赏

