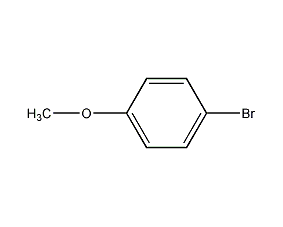
Structural formula
| Business number | 02QZ |
|---|---|
| Molecular formula | C7H7BrO |
| Molecular weight | 187.03 |
| label |
1-Bromo-4-methoxybenzene, p-bromoanisole, p-bromoanisole, 1-Bromo-4-methoxybenzene, p-Bromoanisole, p-Bromophenyl methyl ether, Multifunctional solvents, drug intermediates, aromatic compounds |
Numbering system
CAS number:104-92-7
MDL number:MFCD00000097
EINECS number:203-252-1
RTECS number:BZ8501000
BRN number:1237590
PubChem number:24891850
Physical property data
1. Properties: colorless or light yellow liquid. [1]
2. Melting point (℃): 9~13.5[2]
3. Boiling point (℃) :215~223[3]
4. Relative density (water=1): 1.46[4]
5 .Relative vapor density (air=1): 1.49[5]
6. Octanol/water partition coefficient: 3.05[6]
7. Flash point (℃): 94[7]
8. Solubility: insoluble in water, soluble in ethanol, ether and chloroform. [8]
9. Refractive index at room temperature (n25): 1.571727
10. Relative density (25℃, 4℃): 1.474135
Toxicological data
1. Acute toxicity[9]
LD50: 3800mg/kg (rat oral); 2200mg/kg (mouse oral Oral)
2. Irritation No information available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 40.62
2. Molar volume (cm3/mol): 129.6
3. Isotonic specific volume (90.2K ): 314.4
4. Surface tension (dyne/cm): 34.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.10
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 77
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units:1
Properties and stability
1. Stability[10] Stable
2. Incompatible substances[11] Strong oxidants, strong acids
3. Conditions to avoid contact[12] Heating
4 .Polymerization hazard[13] No polymerization
5. Decomposition products[14] Hydrogen bromide
Storage method
Storage Precautions[15] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of p-bromophenol and dimethyl sulfate. Dissolve p-bromophenol in dilute sodium hydroxide solution, cool to below 10°C, and then slowly add dimethyl sulfate while stirring. The reaction temperature can be raised to 30°C, heated to 40-50°C and stirred for 2 hours. The oil layer is separated, washed with water until neutral, dried with anhydrous calcium chloride, and distilled to obtain the finished product.
2. Use anisole as raw material, carry out bromination reaction with bromine in glacial acetic acid, and finally wash it; obtain it by distillation under reduced pressure.
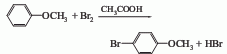
Purpose
1. Used as solvent and in organic synthesis. [16]

 微信扫一扫打赏
微信扫一扫打赏

