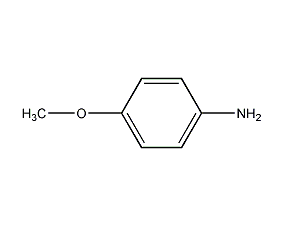
Structural formula
| Business number | 02R1 |
|---|---|
| Molecular formula | C7H9NO |
| Molecular weight | 123 |
| label |
4-Methoxyaniline, 4-Methoxyaniline, p-Aminoanisole, 4-Methoxybenzenamine, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:104-94-9
MDL number:MFCD00007864
EINECS number:203-254-2
RTECS number:BZ5450000
BRN number:471556
PubChem number:24856001
Physical property data
1. Properties: Molten crystals or white crystals, industrial products are yellow to reddish crystals.
2. Density (g/mL, 25℃): 1.064
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 57.2
5. Boiling point (ºC, normal pressure): 243
6. Boiling point (ºC, mmHg): Undetermined
7. Refractive index (n60D): 1.5559
8. Flash point (ºC): 122
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20.2ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
p>
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient Logarithmic value of p>
19. Solubility: Soluble in ethanol, acetone, benzene and ether, slightly soluble in water. Its hydrochloride is easily soluble in water, while its sulfate is insoluble in water. Gradually oxidizes to brown in the air.
Toxicological data
1. Acute toxicity: Mouse oral LD5O: 1410mg/kg
Rat oral LD5O: 1320mg/kg
Rabbit oral LD5O: 2900mg/kg
p>
Rat transdermal LD5O: 3200mg/kg
Ecological data
It is extremely harmful to water and toxic to fish. Do not let the product enter the water body.
Molecular structure data
1. Molar refractive index: 37.16
2. Molar volume (cm3/mol): 115.7
3. Isotonic specific volume (90.2K): 289.7
4. Surface tension (dyne/cm): 39.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 35.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 77
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants. This product is highly toxic. The maximum allowable concentration in the air is 0.5mg/m3. For protective methods, see anthranilate.
2. It is irritating to the respiratory system, so avoid inhaling the vapor of this product.
3. Exist in smoke.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. Packed in iron drums, with a net weight of 200kg per drum. Store in a ventilated, dry place, away from sunlight. Store and transport according to regulations on toxic and dangerous goods.
Synthesis method
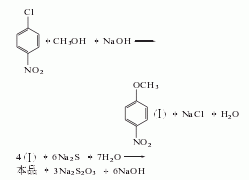
1. The raw materials of the traditional sodium sulfide method are p-nitrochlorobenzene and methanol; liquid caustic soda performs a methoxylation reaction to generate p-nitroanisole, and sodium sulfide is used to reduce p-nitroanisole, and the reaction product is separated ; After distillation under reduced pressure, the finished product p-aminoanisole is obtained. Add 23% sodium sulfide to the reduction pot, stir and heat to 90°C, add p-nitroanisole within 4 hours, and control the feeding temperature at 90-95°C. After the addition is completed, the temperature is raised to 100°C and stirred for 0.5h. The temperature is raised to 110-111°C and stirred for 2h. The waste water is separated and distilled under reduced pressure to obtain p-methoxyaniline.
Raw material consumption quota: p-nitrochlorobenzene 1348kg/t; methanol 398kg/t; solid alkali 367kg/t; alkali sulfide 1102kg/t.
2. Catalysis Hydrogenation-reduction method Hydrogenation-reduction method is commonly used to produce p-aminoanisole abroad, and my country is also beginning to experiment with new hydrogenation-reduction processes.
Purpose
1. This product is mainly used in the dye industry to prepare ice dyes, such as maroon red base GP, blue salt VB, naphthol AS-RL, naphthol AS-SG, C.I. disperse blue 79 and other dyes and anti-inflammatory Intermediates for pharmaceuticals such as painkillers, adipine, and primaquine.
2. Biochemical research, organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

