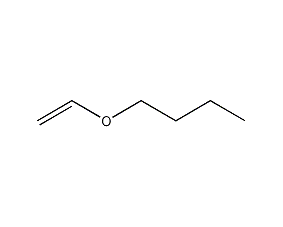
Structural formula
| Business number | 033B |
|---|---|
| Molecular formula | C6H12O |
| Molecular weight | 100.16 |
| label |
vinyl n-butyl ether, vinyl n-butyl ether, 1-(Ethyloxy)butane, butyl vinyl ether, vinyl butyl ether, 1-(Ethenyloxy)-butane, 1-(Vinyloxy)-butane, (Butyloxy)ethylene, Vinyl butyl ether, adhesive, additives, plasticizer, straight chain compounds, Electronic coating raw materials and intermediates |
Numbering system
CAS number:111-34-2
MDL number:MFCD00009454
EINECS number:203-860-7
RTECS number:KN5950000
BRN number:1560217
PubChem number:24846240
Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 20/20℃): 0.7803
3. Relative vapor density (g/mL, air =1): 3.45
4. Melting point (ºC): -92
5. Boiling point (ºC, normal pressure): 93.8
6. Refractive index (n20ºC): 1.4026
7. Refractive index (n25ºC): 1.3997
8. Flash point (ºC, open): -9.5
9. Viscosity (mPa·s, 20ºC): 0.47
10. Vapor pressure (kPa, 65.9ºC): 40.00
11. Vapor pressure (kPa, 20ºC): 5.60
12. Vapor pressure (kPa,-5ºC): 1.33
13. Volume expansion coefficient (K-1, 55ºC): 0.00133
14. Body expansion coefficient (K-1, 10~40ºC): 0.00100
15. Solubility: Slightly soluble in water, able to dissolve in benzene, ether, and carbon tetrachloride Miscible with organic solvents such as hexane and hexane. Dissolves 0.30% in water at 20°C; water dissolves 0.09% in butyl vinyl ether.
16. Relative density (20℃, 4℃): 0.7888
17. Relative density (25℃, 4℃): 0.7742
18. Critical Temperature (ºC): 266.85
19. Critical pressure (MPa): 3.20
20. Critical density (g·cm-3): 0.261
21. Critical volume (cm3·mol-1): 384
22. Critical compression factor: 0.274
p>
23. Eccentricity factor: 0.380
24. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3891.5
25. Gas phase standard Claimed heat (enthalpy) (kJ·mol-1): -184.5
26. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1 sup>): -3857.2
27. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -218.8
28. Liquid Phase standard hot melt (J·mol-1·K-1): 214.1
Toxicological data
1. Acute toxicity: Oral LD50 in rats: 10gm/kg; Inhalation LCLO in rats: 16000ppm/4H
Inhalation LC50 in mice: 62gm/m3/2H; Dermal LD50 in rabbits: 4240ul /kg
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 31.31
2. Molar volume (cm3/mol): 129.0
3. Isotonic specific volume (90.2K ): 279.5
4. Surface tension (dyne/cm): 22.0
5. Polarizability (10-24cm3): 12.41
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 41.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides. It is stable to alkali and prone to hydrolysis in acid. The double bonds in butyl vinyl ether are activated by the influence of alkoxy groups, and can add to a variety of compounds to generate various derivatives. Addition with benzamide produces butanol. Butylene vinyl ether alone does not undergo homogeneous polymerization. Addition occurs first to basic compounds in the presence of a catalyst. In the presence of Friedel-Crafts type acidic catalysts such as aluminum trichloride and boron trifluoride, polymerization reactions can occur. Water is the largest polymerization inhibitor during low temperature polymerization.
Storage method
This product is a first-class flammable liquid. Store sealed in a cool, ventilated and dry place. Ensure that the workspace has good ventilation facilities and explosion-proof facilities. Keep away from fire sources and prevent static electricity. Store away from oxidizing agents.
Synthesis method
1, Acetylene method Obtained from the reaction of acetylene and butanol . This method requires less equipment and is highly selective.
2, acetaldehyde method Obtained from the reaction of acetaldehyde and n-butanol. This method of acetal cleavage is also a common process for vinyl ethers. Another newer method is to react with alcohol; ethylene and oxygen in the presence of palladium chloride–copper chloride catalyst, vinyl ether can also be obtained.
3. Preparation method:
![]()
In equipment equipped with stirrers and fractionation devices In the reaction bottle, add 5g (0.016mol) of mercury acetate, 50g (1.08mol) of absolute ethanol, and 100g (1mol) of n-butyl vinyl ether (2). The receiving bottle and condenser are fully cooled. Heat to reflux, control the distillation temperature of the fractionating column to be 36-37°C, and distill out a total of 71g of ethyl vinyl ether (1). The yield is 98%. After steaming, water was added to the reaction flask and subjected to azeotropic distillation. The boiling point was 97°C, and finally 71.2g of n-butanol was obtained. [1]
Purpose
Organic synthetic raw materials, used to polymerize to produce polyvinyl ether, used in coatings, adhesives, additives, plasticizers, etc.

 微信扫一扫打赏
微信扫一扫打赏

