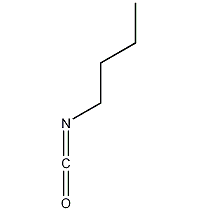
Structural formula
| Business number | 033C |
|---|---|
| Molecular formula | C5H9NO |
| Molecular weight | 99 |
| label |
None yet |
Numbering system
CAS number:111-36-4
MDL number:MFCD00002046
EINECS number:203-862-8
RTECS number:NQ8250000
BRN number:773917
PubChem number:24892145
Physical property data
1. Characteristics: Undetermined
2. Density (g/mL, 20℃): 0.880
3. Relative vapor density (g/mL, air=1) : Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC , kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): 26
9. Specific rotation (º ): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
1. Acute toxicity: Rat oral LD5O: 600mg/kg
Rat inhalation LC5O: 3gm/m3
Mouse oral LD5O: 150mg/kg
p>
Mouse inhalation LC5O: 680mg/m3
Mouse intravenous LD5O: 1mg/kg
Rabbit transdermal LDLO: 6gm/kg
Guinea pig oral LD5O: 250mg/kg
Guinea pig transdermal LD5O: >1gm/kg
2. Other multi-dose toxicity: rat inhalation TCLO: 15mg/m3/6H/ 5D-I
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 29.30
2. Molar volume (cm3/mol): 113.6
3. Isotonic specific volume (90.2K): 265.2
4. Surface tension (dyne/cm): 29.6
5. Polarizability (10-24cm3): 11.61
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 29.4
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 74.1
10. Number of isotope atoms: 0
11.Confirm�Number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
1. Obtained from the reaction of n-butylamine and phosgene. Add n-butylamine and o-dichlorobenzene into the reactor, and pass dry hydrogen chloride gas through it under stirring until it is saturated. Then pass in excess phosgene at 110-160°C until the solution becomes clear, and then pass it through for 20-30 minutes. After completion, distillation, collect the fractions before 160℃, distill again, collect the fractions between 106-120℃, add anhydrous potassium carbonate and let it stand, filter to obtain the finished product. In addition, it can also be prepared by the action of butyl bromide and potassium cyanate in an organic solvent (dimethylformamide).
2. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and ventilation tube (extending into the bottom of the bottle), add n-butylamine (2 ) 73g (1.0mol), 1L o-dichlorobenzene, and stir in dry hydrogen chloride gas until saturated to generate butylamine hydrochloride. Heat and pass phosgene at 110-160°C. After the reaction solution is clarified, continue to pass phosgene for 25 minutes. Distill, collect the fractions before 160°C, re-distill, collect the fractions between 106 and 120°C, dry with anhydrous potassium carbonate, and filter to obtain 70g of butyl isocyanate (1), with a yield of 70%. [1]
Purpose
Organic synthesis intermediate, used in the production of fungicide benzalite.

 微信扫一扫打赏
微信扫一扫打赏

