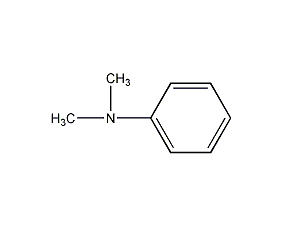
Structural formula
| Business number | 03DX |
|---|---|
| Molecular formula | C8H11N |
| Molecular weight | 121.18 |
| label |
Dimethylaniline, N,N-dimethylaniline, dimethylaniline, N,N-dimethylacetamide, dimethylaminobenzene, Acetyldimethylamine, Xylidine, N,N-Dimethylaniline, Dimethylaniline, metal preservatives, Epoxy resin hardener, Curing accelerator for polyester resin, Cocatalyst for polymerization of ethylene compounds |
Numbering system
CAS number:121-69-7
MDL number:MFCD00008304
EINECS number:204-493-5
RTECS number:BX4725000
BRN number:507140
PubChem number:24864585
Physical property data
1. Characteristics: yellow transparent oily liquid with pungent ammonia smell. [1]
2. Melting point (℃): 2.5[2]
3. Boiling point (℃): 193.1 [3]
4. Relative density (water = 1): 0.96[4]
5. Relative vapor density (Air=1): 4.17[5]
6. Saturated vapor pressure (kPa): 0.13 (29.5℃)[6]
7. Heat of combustion (kJ/mol): -4776.5[7]
8. Critical pressure (MPa): 3.63[8]
9. Octanol/water partition coefficient: 2.31[9]
10. Flash point (℃): 62 (CC)[10]
11. Ignition temperature (℃): 371[11]
12. Explosion upper limit (%) : 7.0[12]
13. Lower explosion limit (%): 1.0[13]
14. Solubility: Insoluble in water, soluble in ethanol, ether, chloroform, acetone, benzene and other organic solvents. [14]
15. Viscosity (mPa·s, 25ºC): 1.528
16. Flash point (ºC): 371
17. Heat of evaporation (KJ/kg, 476.66K): 45.2
18. Heat of fusion (KJ/kg): 97.5
19. Heat of generation (KJ/mol, liquid ): 34.3
20. Heat of combustion (KJ/mol, 20ºC): 4784.3
21. Heat of combustion (KJ/mol, 25ºC, calculated value): 4757.5
22. Specific heat capacity (KJ/(kg·K), 18~64.5ºC, constant pressure): 1.88
23. Boiling point rising constant: 4.84
24. Electrical conductivity (S/m, 20ºC): 2.1×10-8
25. Thermal conductivity (W/(m·K), 20ºC): 0.143
26. Body expansion coefficient (K-1): 0.000854
Toxicological data
1. Acute toxicity[15]
LD50: 951mg/kg (rat oral); 1770mg/kg (rabbit dermal )
2. Irritation [16] Rabbit transdermal: 10mg (24h), mild irritation (open stimulation test)
p>
<h2 id="st"Indoor preparation can react aniline with trimethyl phosphate.
3. Mix aniline and methanol (n aniline: n methanol ≈ 1:3), and inject it into the reactor equipped with the catalyst through a reciprocating pulse-free metering pump at an air speed of 0.5h-1, and the reaction The effluent first enters the glass gas-liquid separator, and the liquid collected at the lower part of the separator is taken out regularly for chromatographic analysis.
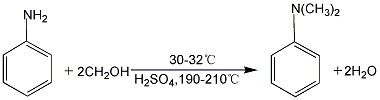
4. In 2001, Nankai University and Tianjin Ruikai Technology Development Co., Ltd. jointly developed a high-efficiency aniline methylation catalyst to achieve gas-phase synthesis of N, N-dimethylaniline. The process is as follows: after liquid aniline is mixed with methanol in proportion, it is vaporized in the vaporization tower, and then enters the tubular reactor with a space velocity of 0.5-1.0h-1 (the fixed bed of the tubular reactor is equipped with a supported nano-solid catalyst). Continuous production at 250-300℃ and normal pressure. The DMA yield reaches over 96%.
Refining method: often contains impurities such as aniline and N-methylaniline. During purification, N,N-dimethylaniline is dissolved in 40% sulfuric acid and steam distilled. Add sodium hydroxide to make it alkaline. Continue with steam distillation. The aqueous layer was separated from the distillate and dried over potassium hydroxide. Carry out atmospheric distillation in the presence of acetic anhydride. The distillate was washed with water to remove trace amounts of acetic anhydride, dried with potassium hydroxide, and then with barium oxide, and distilled under reduced pressure in a nitrogen stream. Other refining methods include adding 10% acetic anhydride and refluxing for several hours to remove primary and secondary amines. After cooling, add excess 20% hydrochloric acid and extract with ether. Add alkali to the hydrochloric acid layer to make it alkaline, and then extract it with diethyl ether. The ether layer is dried with potassium hydroxide and then distilled under reduced pressure under nitrogen flow. N,N-dimethylaniline can also be converted into picrate, recrystallized to a constant melting point and then decomposed with warm 10% sodium hydroxide aqueous solution. Then extract with ether, wash with water, dry and distill under reduced pressure.
5. Mix aniline, methanol and sulfuric acid evenly in proportion, and perform a condensation reaction in an autoclave. After the reaction product is decompressed and methanol is recovered, alkali is added to neutralize, separate and then distilled under reduced pressure to obtain the product. .
6. The methylation reaction between aniline and trimethyl phosphate can produce N, N-dimethylaniline: then extract with ether, dry and distill.
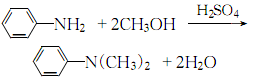
7. In the copper-manganese system Or the mixed vapor of aniline and methanol prepared in a ratio of 1:3.5 can be synthesized by passing the mixed vapor of aniline and methanol at 280°C on the Ziegler catalyst bed of the copper-zinc-chromium system to synthesize N,N-dimethylaniline. The obtained N,N-dimethylaniline is collected through a 54-tray distillation device to collect the fraction between 193-195°C and put into a brown glass bottle. In order to prepare chromatographically pure N,N-dimethylaniline, nitrogen can be used as the carrier gas, and the N,N-dimethylaniline obtained by the above rectification is injected into the preparative gas chromatograph with a benzene column, and collected by separation. The peak fraction of the main component is then put into a glass ampoule and sealed.
Purpose
1. One of the basic raw materials for the production of basic dyes (triphenylmethane dyes, etc.) and basic dyes. The main varieties are basic bright yellow, basic purple 5BN, basic magenta green, and basic lake blue. Brilliant red 5GN, brilliant blue, etc. N,N-dimethylaniline is used in the pharmaceutical industry to manufacture cephalosporin V, sulfonamide-b-methoprim, sulfonamide-b-methoprim, sulfa-dimethoprim, flusporin, etc., and in the spice industry to manufacture vanillin. wait.
2. Used as solvent, metal preservative, epoxy resin curing agent, polyester resin curing accelerator, cocatalyst in the polymerization of vinyl compounds, etc. It is also used to prepare basic triphenylmethane dyes, azo dyes and vanillin, etc.
3. This product is used as a catalyst for producing polyurethane foam in combination with organotin compounds. It is also used as rubber vulcanization accelerator, raw material for explosives and medicine. It is one of the basic raw materials for the production of basic dyes (triphenylmethane dyes, etc.) and basic dyes. The main varieties are basic bright yellow, basic purple 5BN, basic magenta green, basic lake blue, brilliant red 5GN, Brilliant blue etc. N,N-dimethylaniline is used in the pharmaceutical industry to manufacture cephalosporin V, sulfonamide N-methoprim, sulfonamide o-dimethoprim, flusporidine, etc., and in the spice industry to manufacture vanillin, etc. .
4. Used as a curing accelerator for epoxy resin, polyester resin and anaerobic adhesives to quickly solidify the anaerobic adhesives. It can also be used as a solvent, a cocatalyst in the polymerization of vinyl compounds, a metal preservative, a UV absorber for cosmetics, a photosensitizer, etc. It is also used as a raw material for the manufacture of basic dyes, disperse dyes, acid dyes, oil-soluble dyes and spices (vanillin).
5. Used as a reagent for photometric determination of nitrite. Also used as a solvent and used in organic synthesis.
6. Used as dye intermediates, solvents, stabilizers, and analytical reagents. [26]

 微信扫一扫打赏
微信扫一扫打赏

