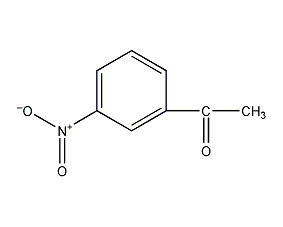
Structural formula
| Business number | 03E7 |
|---|---|
| Molecular formula | C8H7NO3 |
| Molecular weight | 165.15 |
| label |
m-nitroacetophenone, 3-nitroacetophenone, 1-(3-nitrophenyl)ethanone, m-nitroacetophenone, 3-nitroacetophenone, 3′-Nitroacetophenone, 98+%, M-Nitroacetophenone, 3′-Nitroacetophenone, 3-Nitroacetophenone, Akos Bbs-00003220, Akos 233-65, 1-(3-Nitrophenyl)Ethanone, Labotest-Bb Lt01895210, Aurora 14018, Fragrance |
Numbering system
CAS number:121-89-1
MDL number:MFCD00007259
EINECS number:204-504-3
RTECS number:AM9625000
BRN number:743002
PubChem ID:None
Physical property data
1. Characteristics: yellow needle-like crystals.
2. Melting point (℃):81℃
3. Boiling point (ºC,2.0kpa):202℃, 167℃(239kPa).
4. Solubility:Easily soluble in hot water and ether, slightly soluble in ethanol. Can evaporate with water vapor.
Toxicological data
1, acute toxicity: rat oral administrationLD50:3250mg/kg
Mouse transperitoneal cavity LD50: 200mg/kg
Rabbit skinLD50:3mL/kg
2Salmonella mutation test System: 1mg/plate
Ecological data
None
Molecular structure data
5. Molecular property data: 1. Molar refractive index:42.82 2. Molar Volume(m3/mol):132.8 3. Isotonic specific volume(90.2K):347.9 4. Surface tension(dyne/cm)47.1 5. Dielectric constant: 6. Dipole moment(10-24cm3) : 7. Polarizability:16.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 62.9
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 197
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
1. Introduction to production methods
Obtained from the nitration of acetophenone. Additionally, with 2-nitroacetophenone The preparation method is similar, and this product can also be obtained by oxidation of m-nitroethylbenzene.
Purpose
2. Purpose
Used as an intermediate in organic synthesis.
-SIZE: 10pt; COLOR: #333333; FONT-FAMILY: 宋体; mso-ascii-font-family: ‘Times New Roman’; mso-hansi-font-family: ‘Times New Roman'”>from acetophenone Obtained by nitrification. In addition, with 2-nitro The preparation method of acetophenone is similar, and it can also be obtained by oxidizing m-nitroethylbenzene.
Purpose
2. Purpose
Used as an intermediate in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

