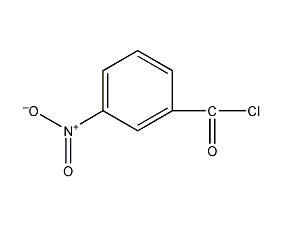
Structural formula
| Business number | 03E8 |
|---|---|
| Molecular formula | C7H4ClNO3 |
| Molecular weight | 185.56 |
| label |
m-nitrobenzoyl chloride, 3-nitrobenzoyl chloride, m-nitrobenzoyl chloride, 3-nitrobenzyl chloride, m-Nitrobenzoyl chloride, 3-Nitro-Benzoylchlorid, Benzoyl Chloride, M-Nitro-, Benzoylchloride,3-Nitro-, Chlorid Kyseliny M-Nitrobenzoove, Chloridkyselinym-Nitrobenzoove, M-Nitro-Benzoylchlorid, 3-Nitrobenzoic Acid Chloride, 3-Nitrobenzoyl Chloride, aromatic compounds |
Numbering system
CAS number:121-90-4
MDL number:MFCD00007247
EINECS number:204-505-9
RTECS number:DM6646000
BRN number:777186
PubChem ID:None
Physical property data
1. Character: yellow or brown liquid(Light yellow needle crystal), has a pungent smell.
2. ; Density (g/mL ,25/4℃):1.428
3. Melting point (℃):37℃
4. Boiling point (ºC):275-278℃(Microdecomposition),164(3.33kPa),154-155(2.93kPa)
5. Solubility:Soluble in ether and benzene, decomposes when exposed to water and ethanol
Toxicological data
1, acute toxicity: rat oral LD50: 2460uL/kg
Rabbit skinLD50:790uL/kg
2Salmonella mutation test System: 100ug/plate
Ecological data
None
Molecular structure data
5. Molecular property data: 1. Molar refractive index:43.04 2. Molar Volume(m3/mol):127.6 3. isotonic ratio(90.2K): 346.5 4. Surface tension(dyne/cm)54.2 5. Dielectric constant: 6. Dipole moment(10-24cm3) : 7. Polarizability:17.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 62.9
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 201
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
1. Introduction to production methods
Originated from the reaction of m-nitrobenzoic acid and phosgene. Mix m-nitrobenzoic acid, benzene and a small amount of dimethylformamide and heat to 40-50℃ , to dissolve them all. Then, phosgene is passed through until the acyl chloride is complete, and then nitrogen is introduced to drive away the remaining phosgene. Distill the reaction product, recover the solvent benzene, and distill it twice under reduced pressure to obtain m-nitrobenzoyl chloride. In addition, m-benzoic acid and thionyl chloride were heated to reflux3h, recover excess thionyl chloride, distill under reduced pressure, yield153-154℃( 1.6kPa) fraction to 90-98%The yield of m-nitrobenzoyl chloride is obtained.
Purpose
2. Purpose
Used as an intermediate for pharmaceuticals and Dangjie color developers.
pt; FONT-FAMILY: 宋体; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial; mso-bidi-font-family: Arial”>Polarizability:17.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 62.9
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 201
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
1. Introduction to production methods
Originated from the reaction of m-nitrobenzoic acid and phosgene. Mix m-nitrobenzoic acid, benzene and a small amount of dimethylformamide and heat to 40-50℃ , to dissolve them all. Then, phosgene is passed through until the acyl chloride is complete, and then nitrogen is introduced to drive away the remaining phosgene. Distill the reaction product, recover the solvent benzene, and distill it twice under reduced pressure to obtain m-nitrobenzoyl chloride. In addition, m-benzoic acid and thionyl chloride were heated to reflux3h, recover excess thionyl chloride, distill under reduced pressure, yield153-154℃( 1.6kPa) fraction to 90-98%The yield of m-nitrobenzoyl chloride is obtained.
Purpose
2. Purpose
Used as an intermediate for pharmaceuticals and Dangjie color developers.

 微信扫一扫打赏
微信扫一扫打赏

