
Structural formula
| Business number | 03E9 |
|---|---|
| Molecular formula | C8H6O4 |
| Molecular weight | 166.13 |
| label |
1,3-phthalic acid, isophthalic acid, phthalic acid, isophthalic acid, Isophthalic acid, Isophthalic acid (position 1,3), isophthalic acid, isophthalic acid, Rarechem Al Bo 0036, M-Phthalic Acid, 1,3-Dicarboxybenzene, 1,3-Phthalicacid, Acide Isophtalique, Acideisophtalique, Acideisophtalique (French), Isophthalate, plasticizer, couplers, Modifier |
Numbering system
CAS number:121-91-5
MDL number:MFCD00002516
EINECS number:204-506-4
RTECS number:NT2007000
BRN number:1909332
PubChem number:24895954
Physical property data
1. Appearance: White crystalline powder or powder crystal
2. Melting point (ºC): 348
3. Solubility: Easily soluble in ethanol, acetone and glacial acetic acid. Slightly soluble in boiling water, hardly soluble in cold water, almost insoluble in benzene and petroleum ether.
4. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3309.1
5. Gas phase standard claimed heat (enthalpy) ( kJ·mol-1): -696.2
6. Standard heat of combustion (enthalpy) of crystalline phase (kJ·mol-1): – 3202.6
7. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -802.99
Toxicological data
This product is toxic. Irritating to skin and mucous membranes. Oral administration of LD5012.2g/kg to rats. Mice were injected with LD504.2g/kg. See Phthalic Anhydride.
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.7
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 74.6
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 179
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
Packed in plastic bags and wooden boxes. Store and transport toxic and flammable chemicals according to regulations.
Synthesis method
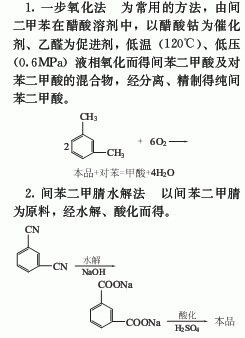
Industrially, isophthalic acid is produced by using m-xylene as raw material, cobalt acetate as catalyst, acetaldehyde as accelerator, acetic acid as solvent, and low-temperature liquid phase oxidation at 120°C under a pressure of about 0.6MPa. Raw material consumption quota: m-xylene (≥95%) 900kg/t, acetaldehyde (≥96%) 600kg/t, acetic acid (≥96%) 500kg/t, cobalt acetate (≥98%) 25kg/t. In addition, hydrolysis of isophthalonitrile or oxidation of m-xylene with potassium permanganate is also an industrial method for producing isophthalic acid.
Purpose
Used in the manufacture of alkyd resins, unsaturated polyester resins, and plasticizers. It is also used in the production of photosensitive materials, polyester fiber modifiers, diagnostic drugs, etc.

 微信扫一扫打赏
微信扫一扫打赏

