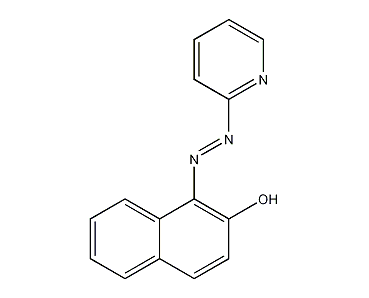
Structural formula
| Business number | 01WB |
|---|---|
| Molecular formula | C15H11N3O |
| Molecular weight | 249.27 |
| label |
α-pyridylazo-β-naphthol;, α-Pyridylazo-β-napnthol, PAN, o-PAN, metal indicator |
Numbering system
CAS number:85-85-8
MDL number:MFCD00004071
EINECS number:201-637-9
RTECS number:None
BRN number:206390
PubChem number:24888068
Physical property data
1. Properties: Orange-red amorphous powder
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/ mL, air=1): Undetermined
4. Melting point (ºC): 141℃
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in methanol, ethanol, benzene, ether, chloroform and hot dilute alkali solution, insoluble in water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 74.04
2. Molar volume (cm3/mol): 199.9
3. Isotonic specific volume (90.2K): 534.0
4. Surface tension (dyne/cm): 50.8
5. Polarizability (10-24cm3): 29.35
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.9
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 54.4
7. Number of heavy atoms: 19
8. Surface charge: 0
9.Complexity: 402
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain atomic stereocenters Quantity: 0
13. Determined number of stereocenters of chemical bonds: 1
14. Uncertain number of stereocenters of chemical bonds: 0
15. Covalent bonds Number of units: 1
Properties and stability
In a solution with a certain pH value, it forms red or other colored complexes with most metal ions. The complexes are insoluble in water.
Storage method
Seal and save.
Synthesis method
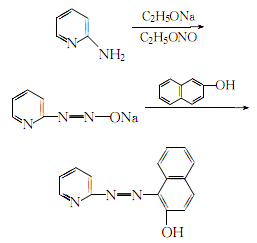
1. Obtained from diazotization of 2-aminopyridine and coupling with 2-naphthol. React absolute ethanol with metallic sodium to generate sodium ethoxide, then add the ethanol solution of 2-aminopyridine, introduce ethyl nitrite vapor and react at 45-50°C. After the reaction is completed, incubate for 8 hours, filter out the diazonium salt and wash with diethyl ether. Add the diazonium salt to the ethanol solution of 2-naphthol, introduce carbon dioxide at 45-50°C for coupling reaction, filter out the crystals after 6 hours, wash with distilled water, recrystallize with ethanol, and dry to obtain the finished product.
2.Add sodium ethoxide obtained by the reaction of absolute ethanol and metallic sodium in the ethanol solution of α-aminopyridine according to the theoretical amount, and then Then introduce ethyl nitrite steam, and control the reaction temperature at 45-50°C. After the steam is introduced, keep it warm for 8 hours. The filtered diazonium salt crystals are washed with diethyl ether. The washed diazonium salt is added to the same amount of In the ethanol solution of 2-naphthol, control the temperature at 45-50°C, then add carbon dioxide for coupling, filter out the crystals after 6 hours, wash with distilled water, recrystallize with 7 times the amount of ethanol, and dry below 80°C , to obtain refined 1-(2-pyridylazo)-2-naphthol.
The process reaction formula is:
Purpose
1. This product is a complex indicator agent. The solution is pink when the pH is above 12, orange-red in weak acid, and purple in concentrated sulfuric acid. Its metal complex is pink or red.
2.Used as a trace metal precipitant to capture titanium, vanadium, manganese, iron, cobalt, nickel, copper, zinc and Tiny trace metals such as lead.

 微信扫一扫打赏
微信扫一扫打赏

