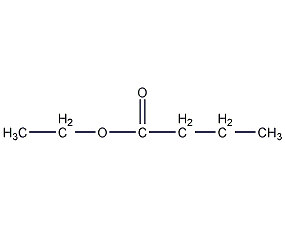
Structural formula
| Business number | 02RV |
|---|---|
| Molecular formula | C6H12O2 |
| Molecular weight | 116.16 |
| label |
Butyric ether, Ethyl butanoate, Butyric n-butyrate, spices |
Numbering system
CAS number:105-54-4
MDL number:MFCD00009394
EINECS number:203-306-4
RTECS number:ET1660000
BRN number:506331
PubChem number:24886845
Physical property data
1. Properties: colorless liquid with pineapple aroma. [1]
2. pH value: ≤1[2]
3. Melting point (℃): -100.8 [3]
4. Boiling point (℃): 120~121[4]
5. Relative density (water =1): 0.879[5]
6. Relative vapor density (air=1): 4.0[6]
7. Saturated vapor pressure (kPa): 1.71 (20℃)[7]
8. Heat of combustion (kJ/mol): -3558.0[8]
9. Critical temperature (℃): 293[9]
10. Critical pressure (MPa): 3.2[ 10]
11. Octanol/water partition coefficient: 1.73 (calculated) [11]
12. Flash point (℃) : 19.44[12]
13. Ignition temperature (℃): 463[13]
14. Explosion upper limit (%): 8.8[14]
15. Lower explosion limit (%): 1.3[15]
16. Solubility: insoluble in water and glycerin, soluble in ethanol and ether. [16]
17. Viscosity (mPa·s, 25ºC): 0.6127
18. Heat of evaporation (KJ/mol, 119ºC): 36.3
19. Heat of formation (KJ/mol): 504.1
20. Specific heat capacity (KJ/(kg·K), 24.1ºC, constant pressure): 1.90
21. Volume expansion coefficient (K-1, 10~30ºC): 0.00116
22. Relative density (25℃, 4℃): 0.8739
23. Critical density (g·cm-3): 0.28
24. Critical volume (cm3·mol-1): 415
25. Critical compression factor: 0.263
26. Eccentricity factor: 0.419
27. Solubility parameter (J·cm-3)0.5: 16.943
28. van der Waals area (cm2·mol-1): 1.049×1010
29. van der Waals volume (cm3·mol-1 ): 73.430
30. Liquid phase standard hot melt (J·mol-1·K-1): 230.4
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 500mg/24Hreaction severity, moderate reaction;
2. Acute toxicity: rat oral LD50: 13050mg/kg ; Rabbit oral LD50: 5228mg/kg; Rabbit skin contact LD50: >2000mg/kg;
3. Olfactory threshold concentration: 0.039mg/m3.
4. Acute toxicity [17]
LD50: 13000mg/kg (rat Oral)
5. Irritation [18]
Rabbit transdermal: 500mg (24h), moderate Stimulate.
Ecological data
1. Ecotoxicity[19]
LC50: 17mg/L (48h) (Goldfish round-bellied yarrow)
IC50: 47~700mg/L (72h) (algae)
2. Biodegradation Properties No information available
3. Non-biodegradability [20] In the air, when hydroxyl When the free radical concentration is 5.00×105/cm3, the degradation half-life is 6 days (theoretical).
When the pH value is 5, 7, 8, and 9, the hydrolysis half-life is 101a, 6.3a, 229d, and 23d respectively (theoretical).
Molecular structure data
1. Molar refractive index: 31.62
2. Molar volume (cm3/mol): 131.0
3. Isotonic specific volume (90.2K ): 295.5
4. Surface tension (dyne/cm): 25.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 12.53
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 68.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: Ethyl butyrate generally does not undergo hydrolysis. However, it is easily hydrolyzed under the action of caustic alkali to produce butyric acid and ethanol. When reacting with sodium hydroxide in 90% methanol, about 90% of ethyl butyrate will be hydrolyzed in 10 minutes at room temperature. When ethyl butyrate is heated to 470~480°C, it decomposes into butyric acid and ethylene. When heated to 465°C in the presence of alumina, it decomposes into carbon dioxide and olefins. When heated to 500°C with ammonia in the presence of alumina, nitrile, ethylene and hydrogen are generated. Ethyl butyrate reacts with propylmagnesium chloride in diethyl ether solution to generate tripropylmethanol and a small amount of dipropylmethylketone. When ethyl butyrate is mixed with acetone, metallic sodium and acetic acid are added, and reduced in ether, 4-octanol-5-one, 2,4-dimethyl-3-propylpentanetriol, 2- Methyl-2-hexanol-3-one. In the presence of sodium ethoxide, ethyl butoxide condenses to obtain 2-butylbutyric acid butyl ester. It reacts with iodine in the presence of manganese or aluminum powder to produce ethyl iodide and manganese butoxide or aluminum butoxide.
2. Stability[21] Stable
3. Incompatible substances[22] Strong oxidants, acids, alkalis
4. Polymerization hazard[23] No polymerization
Storage method
Storage Precautions[24] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Mainly adopt esterification method. It is obtained by using butyric acid and ethanol as raw materials, carrying out acidification reaction in the presence of sulfuric acid catalyst, and then neutralizing, washing, drying and distilling.
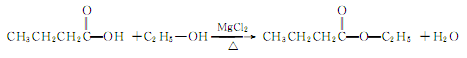
Refining method: often contains butyric acid , ethanol, moisture and inorganic acids and other impurities. During refining, wash with sodium bicarbonate solution and water respectively, dry with anhydrous sodium sulfate or potassium carbonate and then distill. It can also be dried with anhydrous copper sulfate, and then distilled and refined in a dry nitrogen flow.
2. Preparation method:
In a reaction flask equipped with a distillation device, add butyric acid ① (2) 88g (1.0mol), none 92g of water ethanol (2.0mol), one zeolite, 5mL of sulfuric acid. Heat and reflux for 12 hours. Unreacted ethanol was distilled off. Pour the reactant into excess water, separate the organic layer, and wash the organic layer with water and then with saturated sodium bicarbonate solution. Dry over anhydrous sodium sulfate. Distill and collect the fraction at 119-121°C to obtain 87g of ethyl butyrate (1) with a yield of 75%. [26]
Purpose
1. Ester synthetic fragrances. Mainly used as blending spices and masking agent for pear, rose and other flavors. Used as a solvent for glycerol trirosinate, rosin, coumarone resin, eucommia rubber and synthetic resins and a solvent for paints. It is also used as a raw material for pineapple spices and in the manufacture of safety glass.
2. Used in trace amounts in fruity and floral daily flavors, mainly in pineapple, banana, apple and other food flavors and whiskey flavors.
3. Used to prepare food flavors, and can also be used as a solvent for nitrocellulose, many natural or synthetic resins, lacquers, etc.
4. Used as a solvent for cellulose esters and cellulose ethers.
5. Used in the extraction of spices and essences and as solvents. [25]

 微信扫一扫打赏
微信扫一扫打赏

