Background and overview[1]
The scientific name of phenylalanine is α-amino-β-phenylpropionic acid. It is a kind of α-amino acid. It has L type, D type and racemic DL type. The L type is one of the essential amino acids for human body. Phenylalanine was first discovered by Schulze and Barbieri in the late 1880s, a new amino acid isolated from lupine seedlings, and an empirical chemical formula was proposed as C9H11NO2. Erlemeyer and Lipp successfully synthesized phenylalanine through chemical synthesis for the first time in 1882, but their spatial configuration was not explored. Then Fischer accidentally isolated phenylalanine when hydrolyzing casein with hydrochloric acid in 1901. Since then, people have further studied phenylalanine. D-phenylalanine is a kind of α-amino acid. Its physical state is white powder or white crystalline solid at room temperature. Its melting point is 283°C. Its isoelectric point (25°C) is 5.48. Its solubility in water at 25°C is 27g/L. Slightly soluble in methanol, ethanol, insoluble in ether, etc., specific optical rotation [α]25D=+34.5 (c=1.0, H2O). Like other D-amino acids, D-phenylalanine is a very important chiral intermediate in organic synthesis, new drug development, and the synthesis of peptide compounds, and because of the special structure and activity of D-phenylalanine itself Sex is getting more and more attention from people.

Apply[1-3]
Research has found that L-phenylalanine is widely found in nature and is one of the essential amino acids for the human body. It is mainly found in various proteins, such as fibrin and hemoglobin. L-phenylalanine also plays an important role in the secretion of thyroid hormone, hair and skin melanin. As an unnatural amino acid, D-phenylalanine cannot directly participate in human protein synthesis. Its physical and chemical properties are similar to L-phenylalanine. D-phenylalanine has special chiral structure and biological activity. L-phenylalanine has incomparable application value in the fields of industry, agriculture, food, medicine and pesticides. In light industrial production, it has become increasingly common to apply unnatural amino acids to the production of cosmetics to improve and enhance product quality and performance. For example, some manufacturers add amino acids to facial cleansers. The pH of such products is closer to human skin than before, so it is gentle and skin-friendly, non-irritating, and can also protect the skin. In the biochemical reagent industry, since D-amino acids basically do not participate in human metabolism, the half-life of antibiotic drugs with a D-amino acid structure in human metabolism is significantly longer than that of drugs with an L-amino acid structure. Therefore, regarding D-amino acid derivatives in hand The demand for sex drug research is also rising. Among them, biochemical reagents such as N-tert-butoxycarbonyl-D-phenylalanine (BOC-D-phenylalanine) and fluorenylmethoxycarbonyl-D-phenylalanine (FMOC-D-phenylalanine) are all composed of D-phenylalanine. Synthesized by derivatization using amino acid as substrate.
At present, D-phenylalanine is often seen active in the fields of medicine and pesticides. D-phenylalanine can block the activity of enkephalin kinase and inhibit the decomposition of enkephalin, so it is used in enkephalin and enkephalin inhibitors as diuretics and anti-addiction drugs. Drugs synthesized from D-phenylalanine can also be used to prevent and treat Marek’s disease. D-phenylalanine is also partially used in drugs used to treat high blood pressure and diabetes. D-phenylalanine is also widely used as a raw material for drugs to treat arthritis, depression, etc. In addition, there are many other important compounds that require D-phenylalanine to prepare, such as: pyrrolidone peptide compounds, α-aminophosphate carriers for biofilm transmission, AIDS (HIV-1) protease inhibitors, Bombesin receptor antagonists for the treatment of sexual dysfunction, new NEP and ACE inhibitor hydroxamic acid derivatives, N-(amido-alkyl)-4-3-hydroxyphenyl for use as detoxification drugs Piperidine and its derivatives, growth inhibitor analogs for the treatment of gastrinoma, drugs for the prevention and treatment of arteriosclerosis, analgesics, peptide-based antithrombotic agents, neuroleptic drugs, metalloproteinase inhibitors, anti- Fungicides, organotin pesticides, etc. all use D-phenylalanine. At present, D-phenylalanine is mainly used as a raw material for the famous anti-diabetic drug nateglina and the anti-tumor drug Bescin.
Preparation[1]
In the past more than a century, the production and preparation methods of amino acids have been one of the hot spots of research. At present, the research on the production methods of L-phenylalanine is relatively mature, mainly including direct fermentation method, enzymatic method and chemical synthesis method. However, D-phenylalanine is an unnatural amino acid that is difficult to obtain from natural products and cannot be prepared through direct fermentation. The chemical synthesis production process is complex and requires high equipment, making it difficult to produce on a large scale. With the continuous expansion of the application of D-phenylalanine in light industry, medicine, pesticides, food and other fields, the market demand will also continue to increase, so we are looking for D-phenylalanine with simple process and cheap and easily available raw materials. The preparation method of acid has become one of the research directions of chemists. The current methods for preparing D-phenylalanine can be summarized as splitting methods, biological methods and asymmetric chemical synthesis methods. Among them, asymmetric method synthesis, especially asymmetric catalytic hydrogenation method, has shown broad prospects.
1) Splitting method
Racemic phenylalanine is a compound mixed with equal amounts of L-phenylalanine and D-phenylalanine. Their physical and chemical properties are basically the same.They just have opposite optical rotations. Therefore, it is difficult to separate the racemate into a single configuration of phenylalanine using general physical methods, and special methods must be used for separation. Using 3,5-dinitrobenzoyl-substituted β-cyclodextrin as the stationary phase, chiral thin layer chromatography was used to resolve D and L-amino acids. The experiment found that due to the presence of phenylalanine in the benzene ring structure It interacts strongly with the chiral stationary phase, causing racemic phenylalanine to have different physical properties, thus achieving better resolution results. However, the resolving agent used in this method is expensive, consumes a lot, and has low resolving efficiency, so it is difficult to prepare for large-scale production. Penicillin acylase is immobilized, and then the racemic phenylalanine derivatives N-phenylacetyl-D and L-phenylalanine are separated to obtain crude N-phenylacetyl-D-phenylalanine. The protecting group was removed, and then D-phenylalanine was obtained by cationic resin exchange and desalting treatment, with an optical purity of 91.4% and a yield of 67%. This method also has shortcomings such as low efficiency, high energy consumption, low yield, and low optical purity of the product. There are also relevant literature reports on the use of chemical reagents such as dioxaphosphenes to resolve racemic phenylalanine, but the resolution rate was less than 36%.
2) Biological law
Biological methods have been widely concerned and highly valued in the field of amino acid production due to their relatively mild reaction conditions and high stereoselectivity. There are also many literature reports on the use of biological methods to produce D-phenylalanine. D-configuration amino acid acylase is induced from two special bacteria. This enzyme can be used to convert N-acetyl-D-phenylalanine into D-phenylalanine with a maximum yield of 83.1%. The optical purity of the product has not been reported. Although this method is feasible, it is limited to laboratory research and does not yet have the conditions for industrial production. According to relevant reports, Japan’s Nakahara Chemical Company recombined D-hydantoinase and N-carbamamidoamine into E. coli through genetic engineering methods and successfully expressed them. The crude enzyme was extracted and immobilized to produce D-phenylalanine. Good experimental results have been achieved. At present, companies such as Japan’s Nakamoto Chemical have improved bacterial strains through genetic engineering methods. However, there are still shortcomings in D-phenylalanine production such as low substrate concentration, long conversion time, and low conversion rate.
3) Asymmetric synthesis
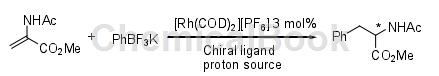
Asymmetric synthesis generally refers to a type of reaction that uses special chemical reagents, solvents, catalysts or physical factors to convert latent chiral units into chiral units, thereby producing unequal amounts of stereoisomeric products. Asymmetric synthesis reactions have developed very rapidly in recent years, with remarkable results, and have become one of the most active research fields in organic chemistry. There are many reports of using this method to synthesize phenylalanine. In 1988, it was reported that D-camphor ketone imine, which was condensed between D-camphor and glycine butylacetate, was used as the chiral synthon. D-phenylalanine was synthesized through enantioselective asymmetric synthesis method. The optical purity of the product was as high as The ee value reaches 95%, and the yield reaches 92.7%. However, this method requires strict reaction conditions, high energy consumption and high cost, and is only suitable for small-scale laboratory synthesis research. Another effective asymmetric synthesis of α-amino acids is the use of rhodium-catalyzed tandem 1,4-addition protonation (Michael type addition reaction) to catalyze α,β-dehydrogenated amino acids. The amido acrylate is reacted with an organic metal reagent in the presence of a rhodium catalyst and a chiral phosphine ligand to obtain the corresponding α-chiral amino acid derivative. The ee value of the phenylalanine derivative is 89.5% and it is in L configuration. This method is simple to operate, but the reaction uses too much rhodium catalyst and chiral phosphine ligands, the cost is too high, and the optical purity of the product is not high. It is still in the laboratory research stage, and there are no production reports using this method.
Main reference materials
[1] Research on new asymmetric synthesis process of D-phenylalanine
[2] Preparation of D-phenylalanine by enzymatic transformation of Bacillus megaterium
[3] Preparation ofD-phenylalanine
by asymmetric transformation and resolution

 微信扫一扫打赏
微信扫一扫打赏

