Background and overview[1][2]
4-[4-[(5S)-5-(aminomethyl)-2-carbonyl-3-zolidinyl]phenyl]-3-morpholinone is an intermediate for the preparation of rivaroxaban, Rivaroxaban is a new type of anticoagulant that can be taken directly orally. It directly inhibits activated coagulation factor In 2008, rivaroxaban was approved for marketing in Canada and the European Union under the trade name Xarelto. On July 1, 2011, Johnson & Johnson and Johnson & Johnson jointly announced that the anticoagulant drug rivaroxaban had been approved by the FDA for the prevention of deep vein thrombosis (DVT). On November 4, 2011, rivaroxaban was approved by the US FDA for the prevention of stroke or systemic embolism in patients with non-valvular atrial fibrillation. The existing preparation process of rivaroxaban has problems such as low safety, expensive raw materials, and low material recovery. Therefore, there is an urgent need for a rivaroxaban preparation process that is cheap, easy to obtain, easy to operate, has mild reaction conditions, is green and environmentally friendly, and has high yield.
Preparation[1]
4-[4-[(5S)-5-(aminomethyl)-2-carbonyl-3-oxazolidinyl]phenyl]-3-morpholinone is prepared as follows:
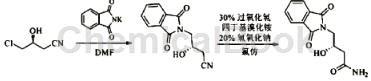
S1. Add phthalimide potassium salt (73.4g, 0.39mol) to a solution of (S)-4-chloro-3-hydroxybutyronitrile (39.5g, 0.33mol) in DMF (330ml) in, heated to 70°C for 4 hours, poured the reaction solution into water (440 ml), stirred for 10 minutes, a white solid precipitated, filtered with suction, and dried the filter cake under reduced pressure to obtain a white solid (73.6g, 97.0%); mp137 ~140℃, [α]D25-21.8°(c1, CHCl3).
S2. Add (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyronitrile (63.4g, 0.28mol) obtained in step S1 to chloroform (330ml ), under ice-water bath conditions, add 30% hydrogen peroxide (122ml, 3.95mol), tetrabutylammonium bromide (17.7g, 55mmol) and 20% sodium hydroxide (110ml, 3.36mol) solution, and complete the addition. Raise to room temperature, stir the reaction for 2 hours, add chloroform (110ml) for separation, wash the organic phase with saturated sodium chloride solution (220ml×3), dry with anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain a white solid 4-[ 4-[(5S)-5-(aminomethyl)-2-carbonyl-3-oxazolidinyl]phenyl]-3-morpholinone (65.6g, 95.8%); mp158~160℃, [α] D25-24.3°(c1, CHCl3).
Main reference materials
[1] (CN108690010) Preparation process of rivaroxaban

 微信扫一扫打赏
微信扫一扫打赏

