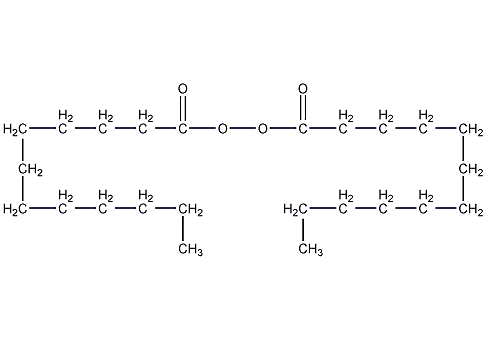
Structural formula
| Business number | 02S8 |
|---|---|
| Molecular formula | C24H46O4 |
| Molecular weight | 398.62 |
| label |
lauroyl peroxide, Lauroyl peroxide, Dilauroyl peroxy, Initiator B, lauroyl peroxide, dodecanoyl peroxide, dilauroyl peroxide, LPO, alperox C, initiator B, Dodecanoyl peroxide, Dilauroyl peroxide, initiator, cross-linking agent, bleach |
Numbering system
CAS number:105-74-8
MDL number:MFCD00008964
EINECS number:203-326-3
RTECS number:OF2625000
BRN number:1804936
PubChem number:24874842
Physical property data
1. Properties: White coarse-grained powder, odorless, with a pleasant smell.
2. Density (g/mL, 25℃): 0.91
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 53~55
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, mmHg): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure ( kPa, 25ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, easily soluble in acetone, chloroform and other organic solvents and minerals Oils. 20. Decomposition temperature (℃): 70~8021. Theoretical oxygen content: 4.02% 22. Activation energy (kJ/mol): 128.7 23. Half-life: 10h (62℃), 3.4h (70℃)
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit eye contact, 500mg/24HREACTION SEVERITY, mild reaction; 2. ChronicToxicity/carcinogenicity: Mouse subcutaneous TDLo: 184mg/kg/46W-I; Mouse route unknown TDLo: 638mg/kg; 3. This product is flammable, and the dust has a strong irritating effect on the eyes, skin and mucous membranes, and can cause burns . LD50 is 184 mg/kg (mouse subcutaneous). Rabbits take 500mg/24h via eyes, mild irritation.
Ecological data
Generally speaking, it is not harmful to water bodies.
Molecular structure data
1. Molar refractive index: 116.78
2. Molar volume (cm3/mol): 431.6
3. Isotonic specific volume (90.2K ): 1036.4
4. Surface tension (dyne/cm): 33.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 46.29
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 10.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 23
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 28
8. Surface charge: 0
9. Complexity: 321
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with metal powder. Stable at room temperature, easily explosive when heated. This product may explode when heated. Contact with reducing agents, accelerators, organic matter, combustibles, etc. will cause violent reactions, posing the risk of combustion and explosion.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from reducing agents, heavy metal compounds, acidic substances, alkali metals, organic materials and metal powders, and avoid mixed storage. Keep away from light. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. Vibration, impact and friction are prohibited.
Synthesis method
1. Preparation method: (1) Add thionyl chloride to lauric acid, heat to 75°C, stir and react for 2 hours, then heat to 90°C and reflux for 2 hours. The reaction mixture is then fractionated, and excess thionyl chloride is first evaporated under reduced pressure, and then the 146-150°C (2.1-2.3kPa) fraction is collected to obtain lauroyl chloride. The yield is about 80%.

2. Preparation method: (2 ) First put lauric acid into the kettle, control the temperature to about 45°C, add phosphorus trichloride dropwise under stirring, and control the dropping time to about 2 hours. During the dropping process, the temperature automatically rises to 55°C. After the dropwise addition, continue the reaction at 55-60°C for a period of time to recover excess phosphorus trichloride to obtain colorless lauroyl chloride liquid.

Dissolve 1 part of lauroyl chloride (volume ) into the reaction kettle, add 0.8 parts of 23.7% sodium hydroxide solution, control the temperature to about 40°C, and add 3 parts of 6% hydrogen peroxide dropwise while stirring. After the dropwise addition, react for 2 to 3 minutes. Cool, add appropriate amount of sulfuric acid to acidify, and then neutralize with potassium hydroxide until neutral. Leave to settle, separate the liquid layer, wash the product with water, filter and dry at low temperature to obtain the finished product of initiator B.
![]()
3. Preparation method:
Lauroyl chloride (3): In a reaction bottle equipped with a stirrer, thermometer, and reflux condenser, add 44.1g (0.22mol) of newly distilled lauric acid (2). 11g of phosphorus trichloride (0.08mol) was stirred and reacted at 50°C for 1 hour. Pour off the upper layer and remove volatile components with a stream of dry air. Distill under reduced pressure and collect the fraction at 138~142℃/2.0kpa to obtain 45.9g of lauroyl chloride with a yield of 95%. Dodecanoyl peroxide (1): Add 100 mL of petroleum ether at 30 to 60°C, an appropriate amount of crushed ice, and 20 g of sodium peroxide into the reaction bottle, then add a solution of compound (3) dissolved in 100 mL of petroleum ether, and shake vigorously for 2 minutes. . Add 15g sodium peroxide and more crushed ice and shake for 15 minutes. Transfer to a separatory funnel, add diethyl ether to allow dodecanoyl peroxide to enter the organic layer. The organic layer was separated, washed with water, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure at room temperature to obtain a waxy white solid (1). Dry in a fast dryer filled with sodium hydroxide to obtain 35.5g of product (1) with a yield of 85%. [1]
Purpose
1. Used as an efficient initiator for polyvinyl chloride and high-pressure polyethylene, often used together with initiator A (di-tert-butyl peroxide). It can also be used as a cross-linking agent for unsaturated polyester, a bleaching agent in the food industry and oil production. 2. As a polymer polymerization initiator and foaming agent, it must be prepared into a 25% white oil solution. It can also be used as a bleaching agent for food and fatty oils.

 微信扫一扫打赏
微信扫一扫打赏

