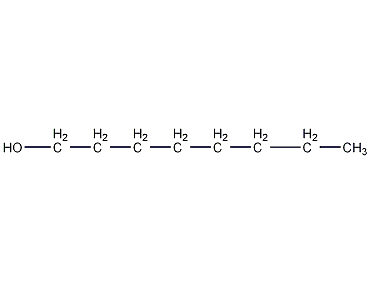
Structural formula
| Business number | 034J |
|---|---|
| Molecular formula | C8H18O |
| Molecular weight | 130.23 |
| label |
Octanol, Subanol, 1-octanol, Caprylic alcohol, Alcohol C8, Capryl alcohol, Octyl alcohol, n-Octanol, Extracting agent, stabilizer, lubricants, Antifreeze exposure, defoamer, Lubricant additives, alcohol solvent |
Numbering system
CAS number:111-87-5
MDL number:MFCD00002988
EINECS number:203-917-6
RTECS number:RH6550000
BRN number:1697461
PubChem ID:None
Physical property data
1. Properties: Colorless and transparent oily liquid with strong oily and citrus smell.
2. Boiling point (ºC, 101.3kPa): 195.2
3. Melting point (ºC): -14.8
4. Relative density (g/mL, 20/4ºC): 0.8258
5. Relative vapor density (g/mL, air=1): 4.48
6. Refractive index (n20ºC): 1.4296
7. Viscosity (mPa·s, 20ºC): 8.93
8. Flash point (ºC, opening): 80
9. Heat of vaporization (KJ/kg): 411.14
10. Heat of combustion (KJ/kg): 40570
11. Specific heat capacity (KJ/(kg·K), 20~30ºC, constant pressure): 2.23
12. Critical temperature (ºC): 385.0
13. Solubility (%, water): 0.01~0.05
14. Thermal conductivity (W/(m·K ), 20ºC): 16.75
15. Freezing point (ºC): -15.2
16. Vapor pressure (kPa, 54ºC): 0.13
17. Body Expansion coefficient (K-1): 0.000828
18. Solubility: Almost insoluble in water, but miscible with alcohol, ether, chloroform, etc.
19. Relative density (25℃, 4℃): 0.8223
20. Refractive index at room temperature (n25): 1.4276
21. Critical pressure (MPa): 2.777
22. Critical density (g·cm-3): 0.262
23. Critical volume ( cm3·mol-1): 479
24. Critical compression factor: 0.254
25. Eccentricity factor: 0.594
26. Solubility parameter (J·cm-3)0.5: 20.733
27. van der Waals area (cm2·mol-1): 1.303 ×1010
28. van der Waals volume (cm3·mol-1): 93.320
29. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): 5364.81
30. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -355.81
31. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -5293.85
32. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -426.77
33. Liquid phase standard entropy (J·mol -1·K-1): 377.4
34. Liquid phase standard free energy of formation (kJ·mol-1): -144.68
35. Liquid phase standard hot melt (J·mol-1·K-1): 325.9
Toxicological data
1. Acute toxicity: LD50: 1790 mg/kg (oral in mice); >3200 mg/kg (oral in rats); >500 mg/kg (dermal in guinea pigs)
2. Octanol is of low toxicity. It is irritating to skin and eyes, but due to low vapor pressure, it is not dangerous when used under normal conditions.
Ecological data
Not harmful to water.
Molecular structure data
1. Molar refractive index: 40.64
2. Molar volume (cm3/mol): 158.0
3. Isotonic specific volume (90.2K ): 367.1
4. Surface tension (dyne/cm): 29.0
5. Polarizability (10-24cm3): 16.11
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 43.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, acids, and acid chlorides. Flammable liquids. Non-corrosive to metals.
2. Chemical reactivity of primary alcohols. Dehydrated to octene under the action of aluminum oxide.
3. In the presence of a catalyst, esterification can occur with acid. Oxidation produces the corresponding aldehyde or acid.
4. Found in flue-cured tobacco leaves.
5. Naturally found in sweet orange oil, pomelo oil and other essential oils.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. 2. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. 3. Equip with corresponding varieties and quantities of fire-fighting equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Octanol exists in free form in essential oils such as bitter orange, pomelo, sweet orange, green tea, violet leaves, etc., or in the form of acetate, butyrate, and isovalerate. In industrial production, octanal can be reduced or prepared by utilizing the octanoic acid present in coconut oil. It can also be prepared by oxo synthesis using heptene-1 as raw material. Heptene, carbon monoxide and hydrogen, in the presence of cobalt salt, generate aldehydes at 150-170°C and high pressure of 20-30MPa. After cobalt removal, they are then pressurized and hydrogenated to primary alcohols using a nickel catalyst. This method has mature production technology abroad.
Refining method: Fractional distillation under reduced pressure, drying with metallic sodium and then fractionating. Or add boron trioxide to reflux and then distill. The distillate is neutralized with sodium hydroxide and then fractionated.
2.Using industrial product n-octanol as raw material, drying and dehydrating with metallic sodium and then distilling under reduced pressure, or adding boron anhydride to reflux and then distilling. The distillate is neutralized with sodium hydroxide and then distilled under reduced pressure. The 98°C fraction is collected at 1333 Pa, which is the pure product.
3. Tobacco: FC, 18, 40; synthesis: using methyl octanoate as raw material, reduction with ethanol and metallic sodium; or purification by fractionation of industrial grade octanol.
4. Preparation method:
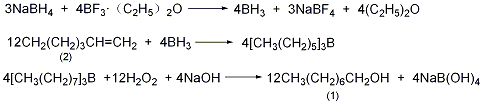
Into a reaction bottle equipped with a stirrer, dropping funnel, thermometer, and ventilation tube, add 50 mL diethylene glycol and 1.7 g (0.045 mol, 20% excess) powdered sodium borohydride, slowly Pour in nitrogen and stir to dissolve. Then, a solution of 1-octene (2) 16.8 (0.15 mol) dissolved in 25 mL of dry diethylene glycol was added. Under cooling in an ice-water bath, add boron trifluoride ether solution 6.5 (48% mass fraction, excess 20%, 0.06 mol) dropwise from the dropping funnel, and continue to slowly add nitrogen for about 30 minutes. During the dropwise addition, keep the temperature of the reaction solution at 20 to 25°C. After the addition, continue stirring for 1 hour to complete the reaction. Add 10 mL of cold water dropwise from the dropping funnel and complete the addition in about 15 minutes. The excess borohydride will be decomposed and a large amount of hydrogen will escape. When no hydrogen gas escapes, add 20 mL of 30% hydrogen peroxide to the dropping funnel. Add 20 mL (3 mol/L) sodium hydroxide solution from the top of the condenser, add hydrogen peroxide dropwise at 30 to 50°C, complete the addition in about 0.5 hours, and continue the reaction at room temperature for 1 hour. Pour the reaction into 70 mL ice water, extract with ether (100 mL × 2), combine the organic layers, wash thoroughly with water (wash 6 times) to remove diethylene glycol, dry over magnesium sulfate, and evaporate the solvent ether. Then separate and collect the 191-193°C fraction. It contains about 7% 2-octanol, the mass is 15.6g, and the yield is 80%. Pure products can be separated using high-efficiency separation columns. The boiling points of 1-octanol (1) and 2-octanol are 194-195°C and 179°C respectively. [1]
Purpose
1. Used as solvent, plasticizer, antifreeze, and also used in making flavors and cosmetics. It is used as a solvent for grease, rubber, resin, polyethylene, butyral, etc., and as a raw material for manufacturing octanoic acid, octyl aldehyde, octyl acetate, plasticizer dioctyl phthalate, cosmetics, and fragrances. Also used as defoaming agent and lubricating oil additive.
2.Used as an additive for oil-based fracturing fluids. It is also used in the production of plasticizers, extractants, stabilizers, and as intermediates for solvents and spices. Octanol itself is also used as a spice to blend rose, lily and other floral essences, and as a soap fragrance.
3. Used for the preparation of daily flavors and fruity food flavors such as carnation, hyacinth, orchids, roses, and cloves.
), combine the organic layers, wash thoroughly with water (wash 6 times) to remove diethylene glycol, dry over magnesium sulfate, and evaporate the solvent ether. Then separate and collect the 191-193°C fraction. It contains about 7% 2-octanol, the mass is 15.6g, and the yield is 80%. Pure products can be separated using high-efficiency separation columns. The boiling points of 1-octanol (1) and 2-octanol are 194-195°C and 179°C respectively. [1]
Purpose
1. Used as solvent, plasticizer, antifreeze, and also used in making flavors and cosmetics. It is used as a solvent for grease, rubber, resin, polyethylene, butyral, etc., and as a raw material for manufacturing octanoic acid, octyl aldehyde, octyl acetate, plasticizer dioctyl phthalate, cosmetics, and fragrances. Also used as defoaming agent and lubricating oil additive.
2.Used as an additive for oil-based fracturing fluids. It is also used in the production of plasticizers, extractants, stabilizers, and as intermediates for solvents and spices. Octanol itself is also used as a spice to blend rose, lily and other floral essences, and as a soap fragrance.
3. Used for the preparation of daily flavors and fruity food flavors such as carnation, hyacinth, orchids, roses, and cloves.

 微信扫一扫打赏
微信扫一扫打赏

