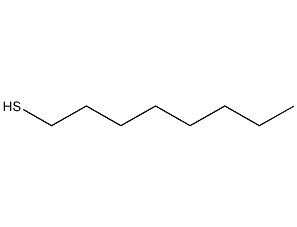
Structural formula
| Business number | 034K |
|---|---|
| Molecular formula | C8H18S |
| Molecular weight | 146.29 |
| label |
Mercaptooctane, Mercapto-n-octane, Octanethiol, octanethiol, 1-octanethiol, thiooctanol, 1-Mercaptooctane, Octane-1-thiol, n-Octyl mercaptan, regulator, additives, Surfactant, stabilizer |
Numbering system
CAS number:111-88-6
MDL number:MFCD00004912
EINECS number:203-918-1
RTECS number:None
BRN number:1733101
PubChem ID:None
Physical property data
1. Properties: water-white liquid with slight odor.
2. Density (g/mL, 20℃): 0.84
3. Relative vapor density (g/mL, air=1): 5.0
4. Melting point (ºC): -49.2
5. Boiling point (ºC, normal pressure): 199.1
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 1.4540
8. Flash point (ºC): 46
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): 85
11. Vapor pressure (mmHg, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 37.7ºC) : 0.21
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure ( KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in alcohol.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2000-2450 mg/kg
Rat skin LD50: 2445mg/kg
Rat inhalation LC50: 4290mg/m3 /4H
Ecological data
Highly toxic to fish.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 47.00
2. Molar volume (cm3/mol): 174.6
3. Isotonic specific volume (90.2K): 402.2
4. Surface tension (dyne/cm): 28.1
5. Polarizability (10-24cm3): 18.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 43.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with alkalis, strong oxidants, strong reducing agents, and alkali metals.
2.Thiols molecules, due to the action of -SH ions, can deteriorate the proteins in the skin and mucous membranes, make the conjunctiva and cornea cloudy and even Dissolve. In addition, it generally has a hypnotic effect and can paralyze the central nervous system at high concentrations. Most thiols can be absorbed through the skin. When applied to the skin, they will cause redness due to irritation in the short term. Long-term exposure can cause cancer. However, as the number of carbon atoms in the thiol molecule increases, its toxicity decreases: C1>C2>C3>C4>C5>C6>C7>C8… The equipment should be sealed and the operators should wear protective equipment. See n-Propanethiol.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
For packaging, storage and transportation methods, please refer to n-propylmercaptan.
Synthesis method
It is derived from the reaction of octyl bromide and sodium hydrosulfide in the presence of sulfuric acid. Octyl mercaptan can also be obtained by condensing octyl bromide and thiourea first, and then hydrolyzing it.
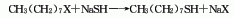
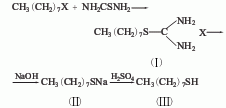
Purpose
This product is used as a polymerization regulator and vulcanization regulator in the production of synthetic rubber, and is also used in the production of rubber additives, medicines, dyes, and pesticides. It can also be used as surfactant and resin stabilizer.

 微信扫一扫打赏
微信扫一扫打赏

