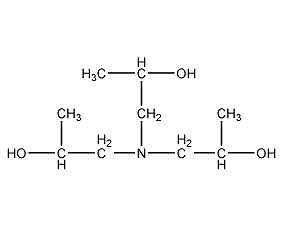
Structural formula
| Business number | 03EQ |
|---|---|
| Molecular formula | C9H21NO3 |
| Molecular weight | 191.27 |
| label |
Triisopropanolamine, 1,1’1”-nitrilotri-2-propanol, 1”-Nitrilotri-2-propanol, Tripropanolamine, 1,1′,1”-Nitrilotri-2-Propanol, 1,1′,1”-Nitrilotripropan-2-ol, 1,1′,1”-Nitrilotris(2-Propanol), Nitrilotripropanol, Tris(2-Hydroxypropyl)Amine, Triisopropanolamine, emulsifier, photographic developer solvent, paraffin oil solvent |
Numbering system
CAS number:122-20-3
MDL number:MFCD00004533
EINECS number:204-528-4
RTECS number:UB8750000
BRN number:1071570
PubChem number:24855404
Physical property data
1. Properties: white crystal or solid powder.
2. Density (g/mL, 50/20ºC): 0.9996
3. Density (g/mL, 60/20ºC): 0.9909
4 . Melting point (ºC): 46
5. Boiling point (ºC): 305.4
6. Viscosity (mPa·s, 60ºC): 138
7. Flash point (ºC, open): 151.7
8. Vapor pressure (kPa, 20ºC): 1.33
9. Solubility: soluble in water, ethanol, ether, etc.
Toxicological data
1. Acute toxicity: Rat oral LD50: 6500mg/kg; mouse oral LD50: 2520mg/kg;
Rabbit oral LD50: 11000mg/kg; Rabbit skin LDLO: 10mL/ KG;
Guinea pigs LD50: 1580mg/Kg; unknown mammalian skin TCLO:> 1gm/kg.
2. Other multiple dose toxicity: oral TDLO in rats: 28gm/kg/2W-C
Ecological data
None
Molecular structure data
1. Molar refractive index: 51.94
2. Molar volume (cm3/mol): 177.9
3. Isotonic specific volume (90.2K): 458.2
4. Surface tension (dyne/cm): 43.9
5. Dielectric constant:
6. Dipole moment (10 -24cm3):
7. Polarizability: 20.59
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.5
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 63.9
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 108
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 3
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Chemical properties are similar to triethanolamine. Isopropyl alcohol is formed during reduction with Raney nickel.
Storage method
None
Synthesis method
Produced by the reaction of propylene oxide and ammonia, it is a co-product of the production of isopropanolamine.
Purpose
Used as pharmaceutical raw materials and photographic developer solvents. Used as a solvent for paraffin oil in the man-made fiber industry. Since the salts formed by triisopropanolamine and long-chain fatty acids have good coloring stability, they are particularly suitable as emulsifiers for cosmetics.

 微信扫一扫打赏
微信扫一扫打赏

