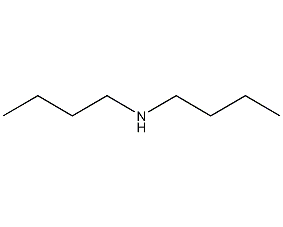
Structural formula
| Business number | 034N |
|---|---|
| Molecular formula | C8H19N |
| Molecular weight | 129 |
| label |
di-n-butylamine, di-n-butylamine, N-Dibutylamine, Di-n-butylamine, n-Butyl-1-butanamine |
Numbering system
CAS number:111-92-2
MDL number:MFCD00009429
EINECS number:203-921-8
RTECS number:HR7780000
BRN number:506001
PubChem ID:None
Physical property data
1. Properties: colorless liquid with ammonia odor. [1]
2. Melting point (℃): -62~-59[2]
3. Boiling point ( ℃): 159~160[3]
4. Relative density (water=1): 0.76[4]
5. Relative vapor density (air=1): 4.46[5]
6. Saturated vapor pressure (kPa): 0.27 (20℃)[6]
7. Critical pressure (MPa): 3.11[7]
8. Octanol/water partition coefficient: 2.83 [8]
9. Flash point (℃): 51.6 (OC) [9]
10. Ignition temperature (℃ ): 312.22[10]
11. Explosion upper limit (%): 10.0[11]
12. Explosion lower limit (%): 1.1[12]
13. Solubility: Slightly soluble in water, soluble in ethanol, ether, acetone, and benzene. [13]
14. Refractive index (20ºC): 1.4177
15. Viscosity (mPa·s, 20ºC): 0.95
16. Heat of evaporation (KJ/mol, 25ºC): 50.58
17. Heat of formation (KJ/mol): -158.14
Toxicological data
1. Acute toxicity[14] LD50: 220mg/kg (rat oral); 1010mg/kg (rabbit dermal)
2. Irritation[15] Rabbit transdermal: 500mg, moderate irritation (open stimulation test)
Ecological data
1. Ecotoxicity[16]
LC50: 5.5mg/L (96h) (rainbow trout, soft water); 37mg/L (96h) (rainbow trout, hard water)
EC50: 87mg/L (24h), 66mg/L (48h) (Daphnia); 16mg/L (72h) (Scenedesmus); 19mg/L (96h) (green algae, static)
2. Biodegradability No data available
3. Non-biodegradability [17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 4h (theoretical ).
4. Bioconcentration[18] BCF: 30 (theoretical)
Molecular structure data
1. Molar refractive index: 42.70
2. Molar volume (cm3/mol): 169.4
3. Isotonic specific volume (90.2K ): 378.3
4. Surface tension (dyne/cm): 24.8
5. Polarizability (10-24cm3): 16.93
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): None
2. Hydrogen�Number of donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 6
5. Tautomerism Number of bodies: None
6. Topological molecule polar surface area 12
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 37.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. No Determine the number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: It has the chemical properties of secondary amines. It is relatively stable against heat and does not change when heated (200~270℃) under reduced pressure (13.33~33.33KPa) for a long time. However, when heated together with aluminum chloride, etc., it will decompose and generate butylamine, ammonia, etc.
2. Stability[19] Stable
3. Incompatible substances[20] Acids, acid chlorides, acid anhydrides, strong oxidants, carbon dioxide
4. Polymerization hazards [21] No polymerization
Storage method
Storage Precautions[22] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
There are two production methods for di-n-butylamine.
1. n-butanol method
It is obtained by the interaction of n-butanol and ammonia. The reaction equation is as follows: n-C4H9OH+NH3+H2→n-C4H9NH2+(n-C4H9)2 NH+(n-C4H9)3N
The process is: pass n-butanol, ammonia and hydrogen through the flow meter Control, and flow into the stainless steel reaction tower according to the proportion. The tower is filled with copper-nickel acidic clay catalyst, and the reaction temperature is around 200°C. After the reaction is completed, the reaction gas is condensed, and the crude product obtained contains 2% to 4.82% of monobutylamine, 17% to 26% of dibutylamine, and 31% to 61% of tributylamine. Monobutylamine, Dibutylamine and tributylamine.
2. Lime nitrogen (calcium cyanamide) method
The reaction equation is as follows: CaCN[NaOH]→Na2NCN[C4H9Br]→(C4H9)2NCN[H2 sub>O]→[H2SO4](C4H9)2NH
The process is as follows: suspend lime nitrogen in ice water, slowly add cooled sodium hydroxide solution, keep the temperature below 25°C, and stir vigorously for 1 hour to obtain sodium cyanamide solution. Add bromobutane ethanol solution to this solution, stir and reflux for 2.5 hours, and then perform distillation. The residue is cooled and filtered with suction. The filter cake is washed with ethanol. The filtrate and washing liquid are combined, extracted with benzene, and the extract is washed with anhydrous water. The calcium sulfate is dried, and after benzene is evaporated, the residue is distilled under reduced pressure, and the 147-151°C/4.66kPa fraction is collected to obtain dibutyl cyanamide. Add water, concentrated sulfuric acid, and dibutyl cyanamide to the reactor in turn, reflux slowly for 6 hours, cool, and pour cooled sodium hydroxide solution along the wall of the reactor (flow to the bottom of the reactor without mixing with the reaction materials) to free the amine. Separate; then heat to evaporate the amine and water together. Add granular potassium hydroxide to the distillate, cool it with ice, separate the amine layer, dry and distill, and collect the 157-160°C fraction to obtain the finished product.
Purpose
1. Used as corrosion inhibitors, emulsifiers, insecticides, polymerization inhibitors, medicines, pesticides, dyes, flotation agents, anti-corrosion agents, plasticizers, rubber vulcanization accelerators, etc.
2. Used as corrosion inhibitor, emulsifier, rubber accelerator, insecticide, polymerization inhibitor, etc. [23]

 微信扫一扫打赏
微信扫一扫打赏

